Supplier Qualification software
Improve the quality of your suppliers
The qualification of suppliers is essential to minimise the risks of outsourcing services and at the same time ensure the quality of products across global supply chains.
- Supplier status
- Compliant with standards
- Efficient & Transparent
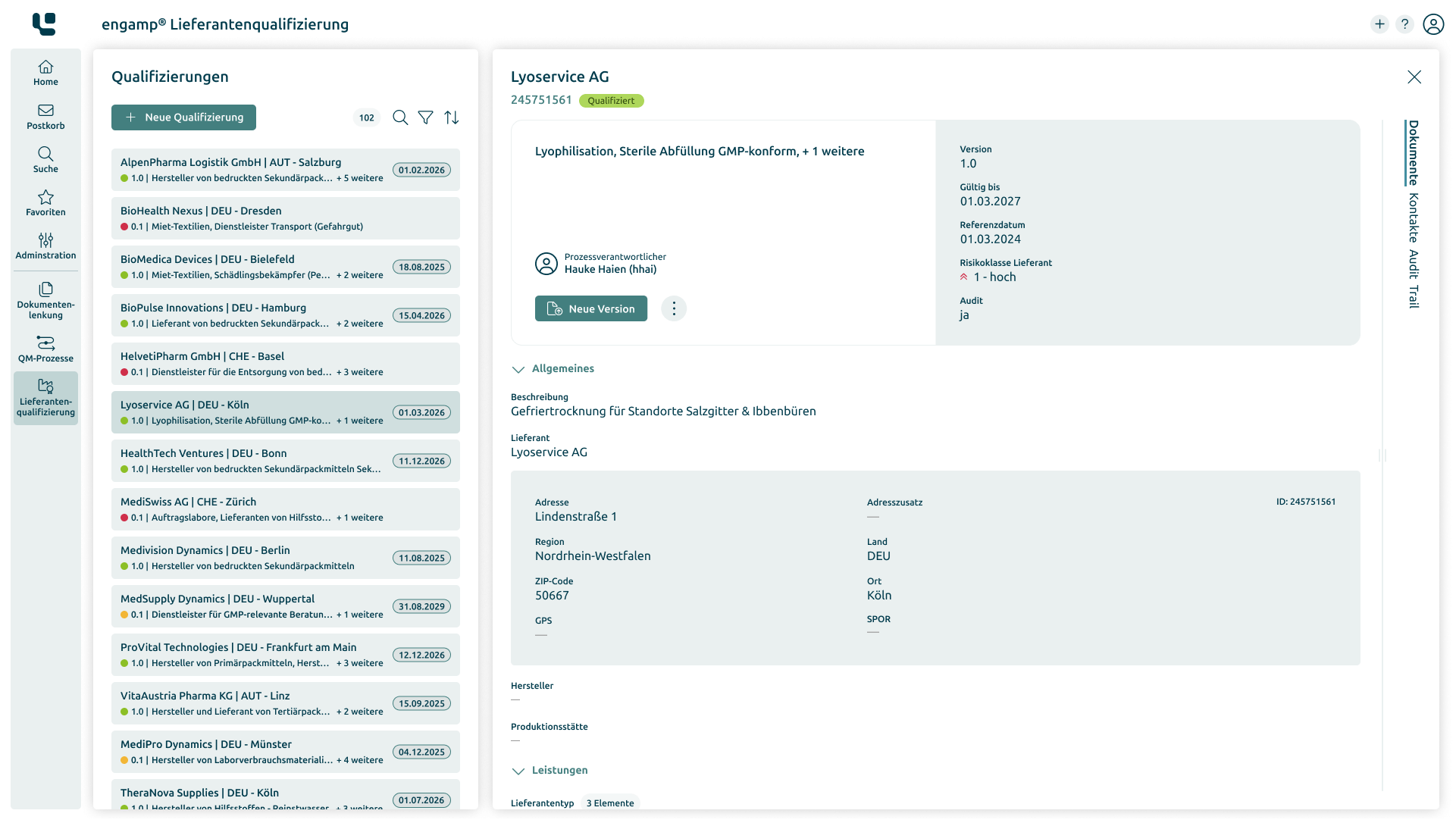
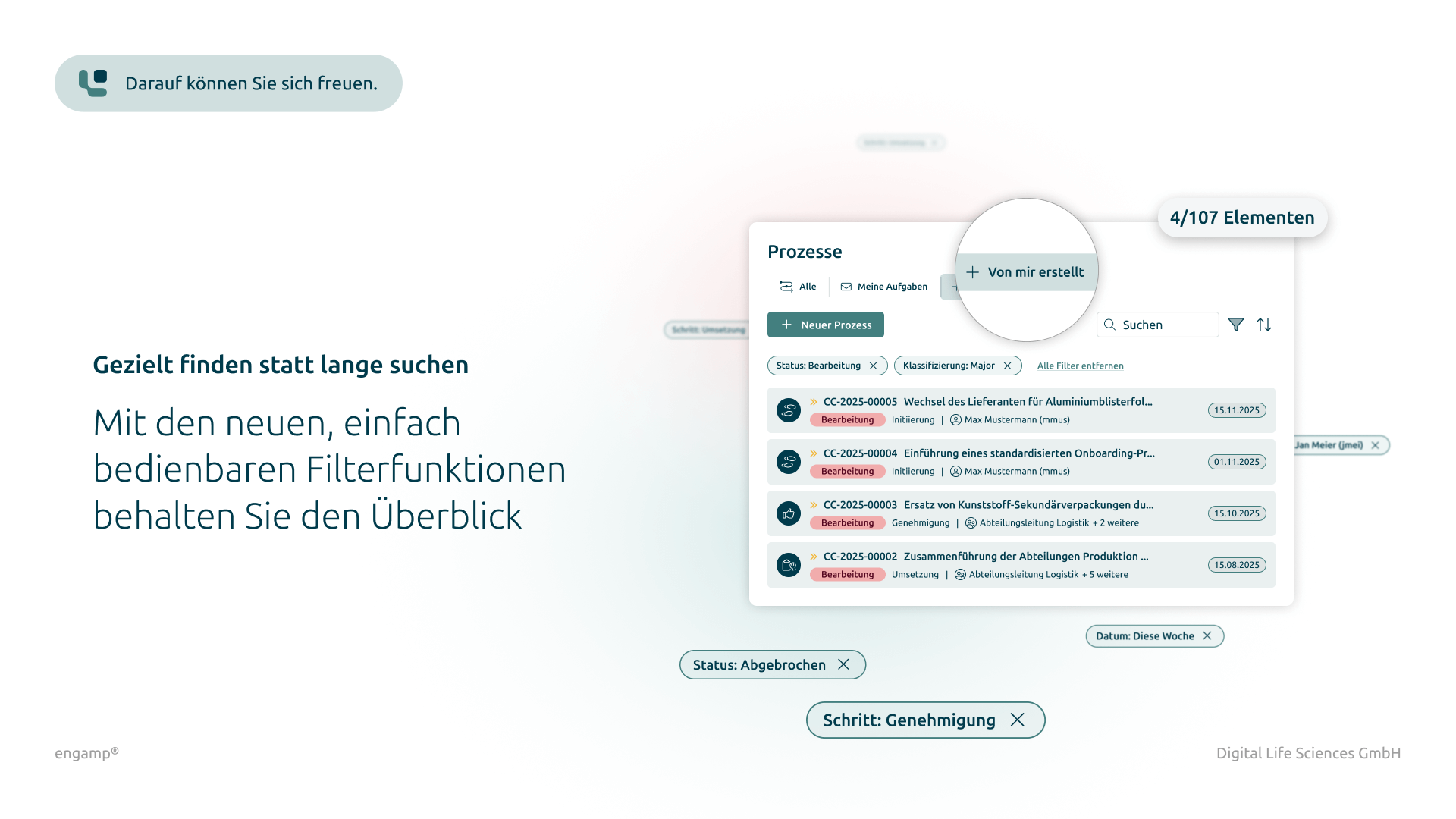
How does supplier qualification help?
Software for qualifying your suppliers
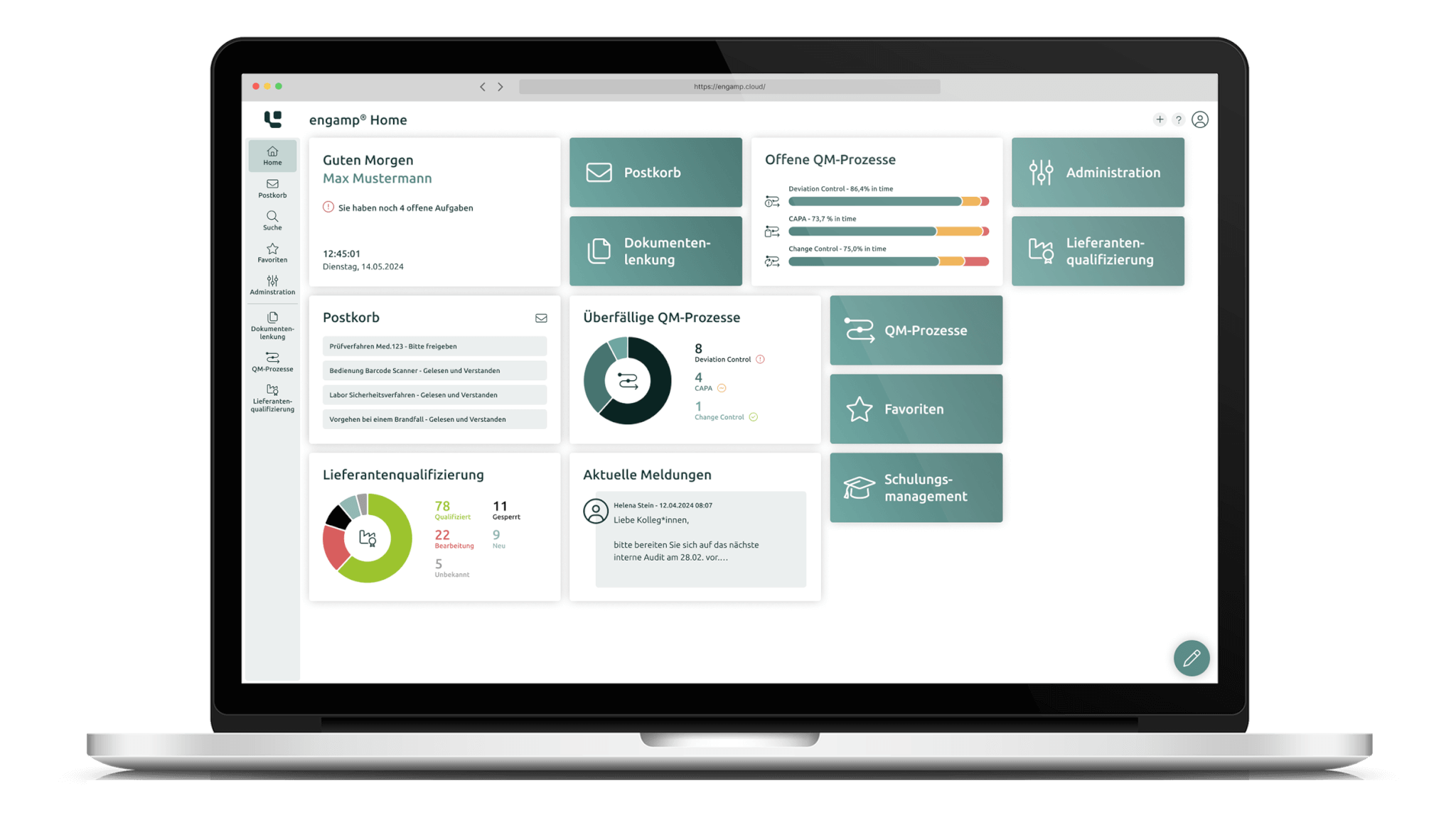
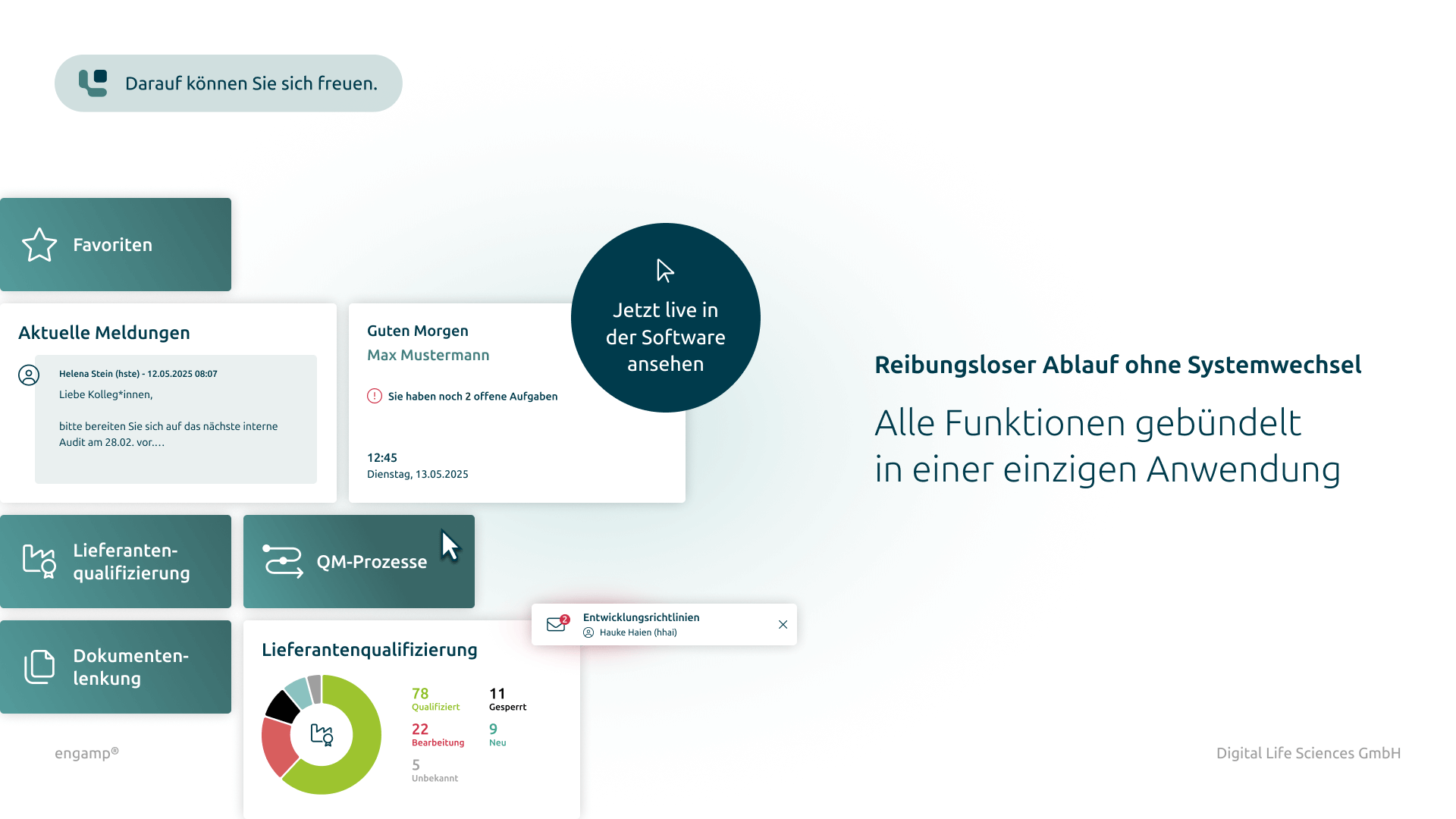
The software “engamp® | supplier qualification” digitises and optimises supplier qualification, an essential component of quality management. It documents risk-based evaluations and always provides up-to-date overviews of the suppliers' qualification status. Centralised data provision and automated qualification processes enable fast, well-founded decisions and reduce manual tasks. The supplier qualification software improves your audit planning, monitors certificate validity, and minimises quality risks through transparent processes and automated workflows.

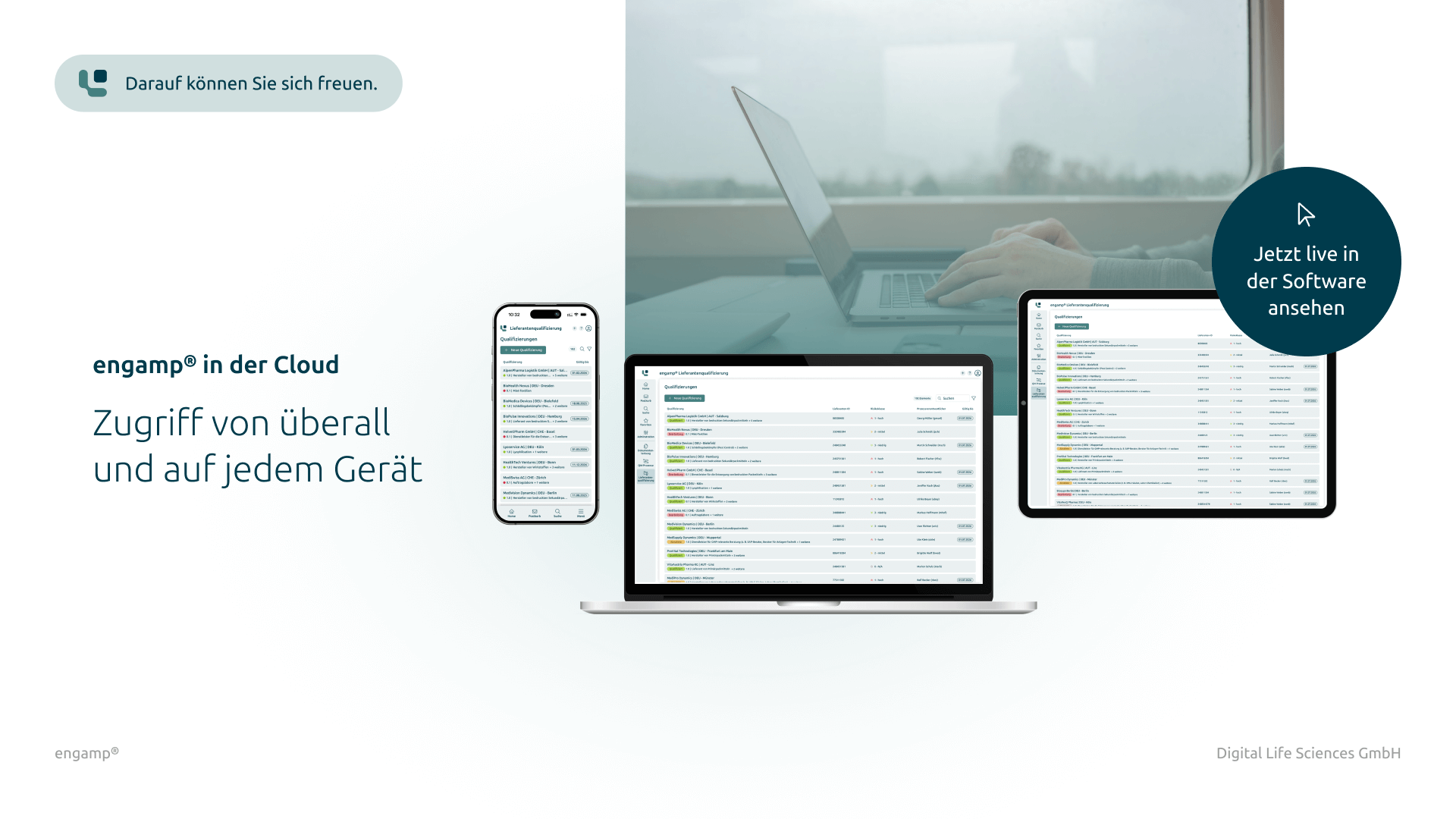
Advantages of supplier qualification
Advantages of the supplier qualification software
The software engamp® | supplier qualification offers comprehensive functions for the structured and efficient qualification of suppliers. It ensures transparent documentation, automated workflows and effective risk evaluation.
01.
Improved decision-making
A central source of information enables well-founded decisions to be made on the approval of suppliers for certain materials and services.
02.
Increased transparency
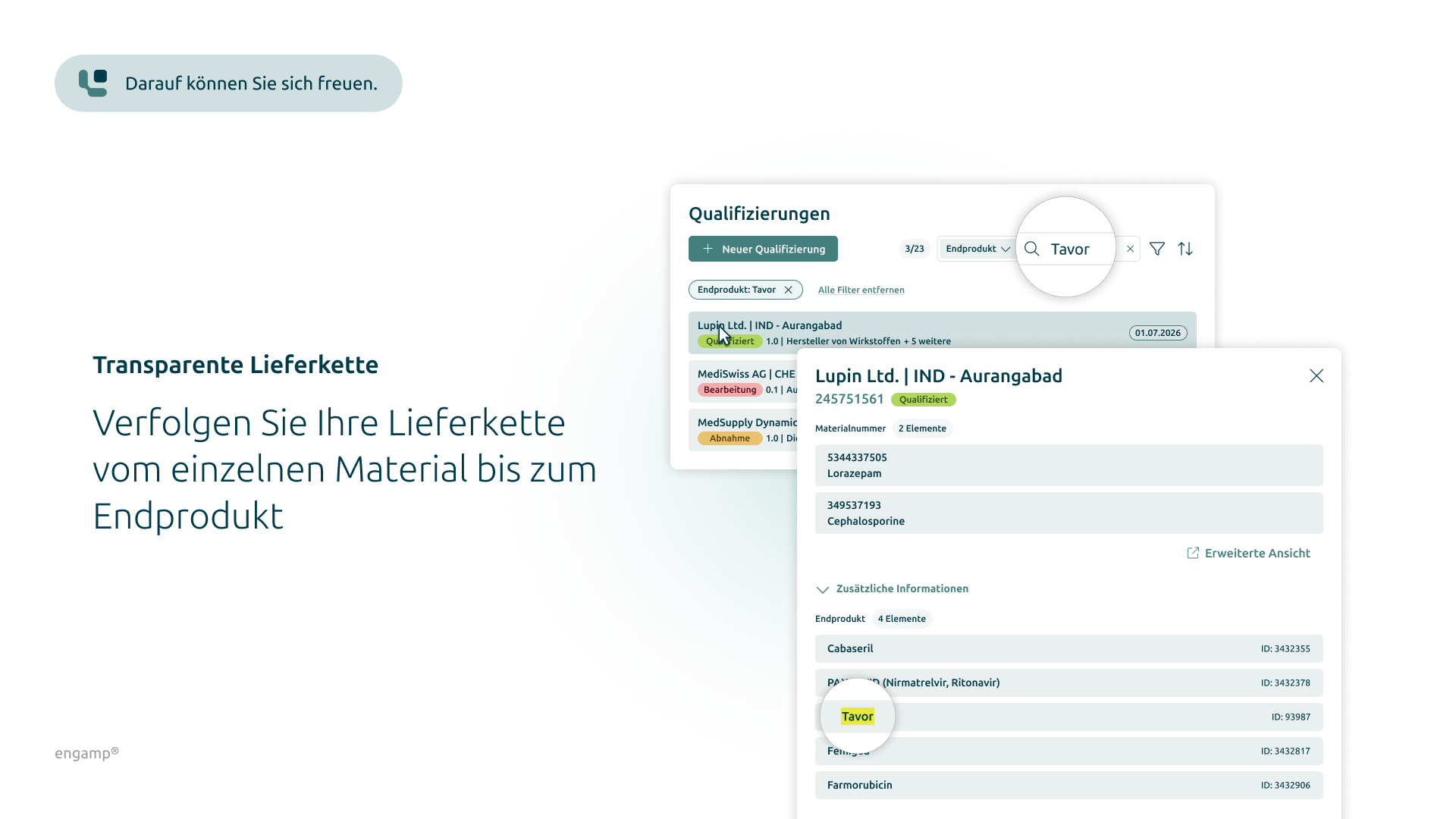
The visual representation of the entire supply chain – from individual materials to the end product – creates transparency for all stakeholders. A detailed examination by target country enables a targeted analysis and optimisation of the supply chain.
03.
Risk-based definition of qualification measures
The software supports the definition of the required documents on the basis of a risk evaluation. This ensures that all regulatory requirements are met and that no essential proof is missing.
04.
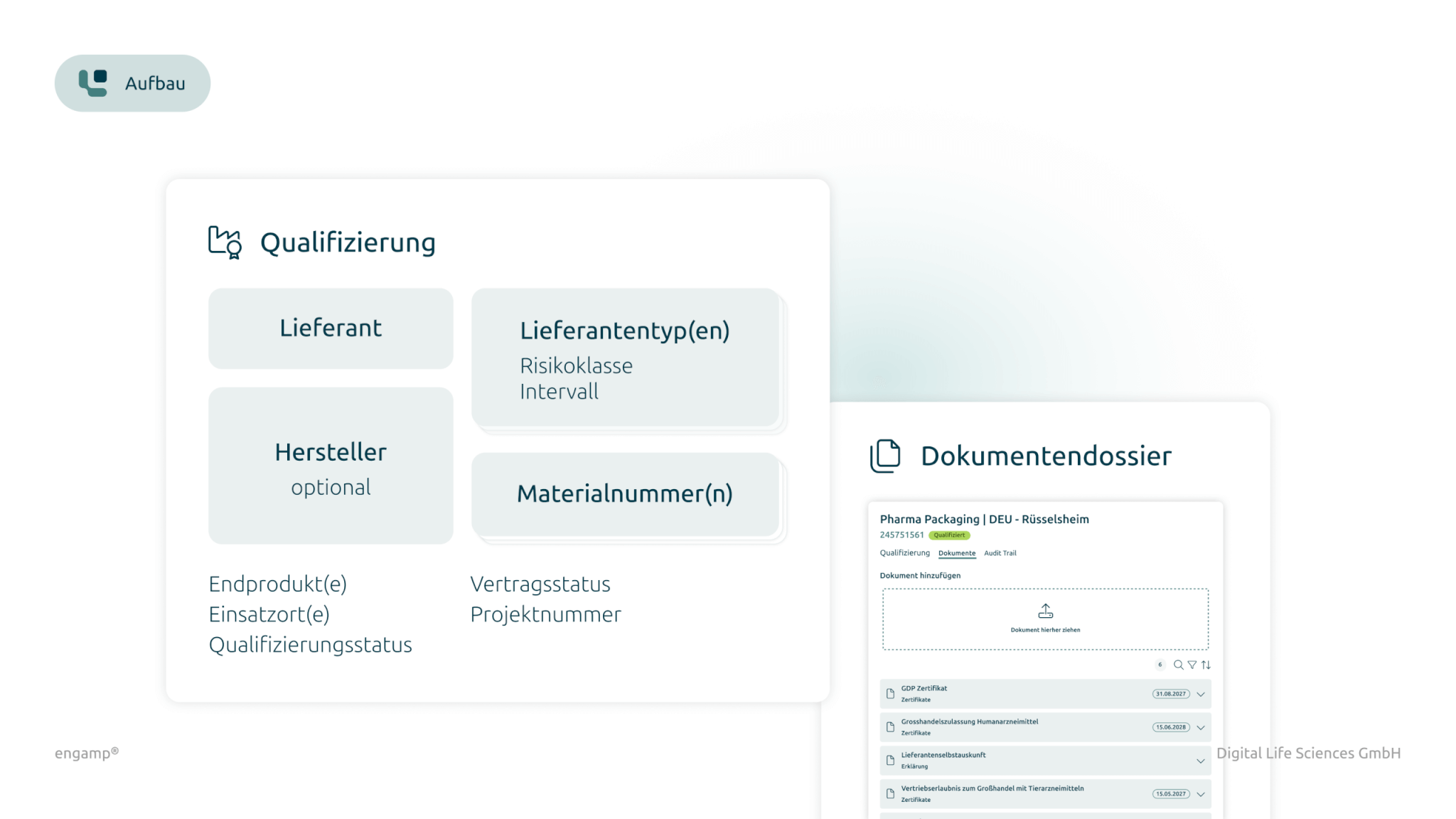
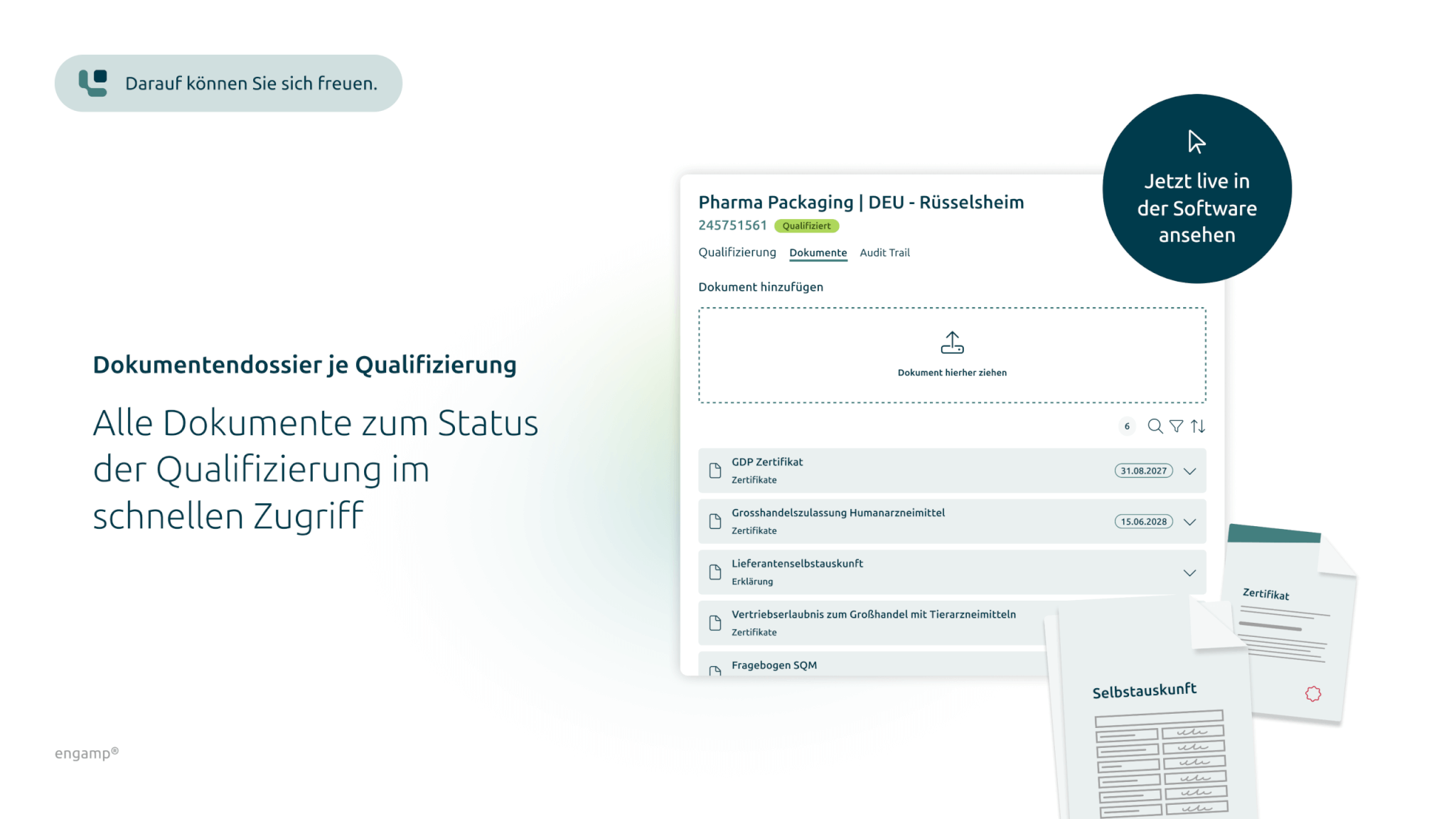
Specific documentation dossier
Each supplier qualification is documented with a specific document dossier containing all relevant certificates, contracts, and proof to ensure a comprehensive and up-to-date overview.
05.
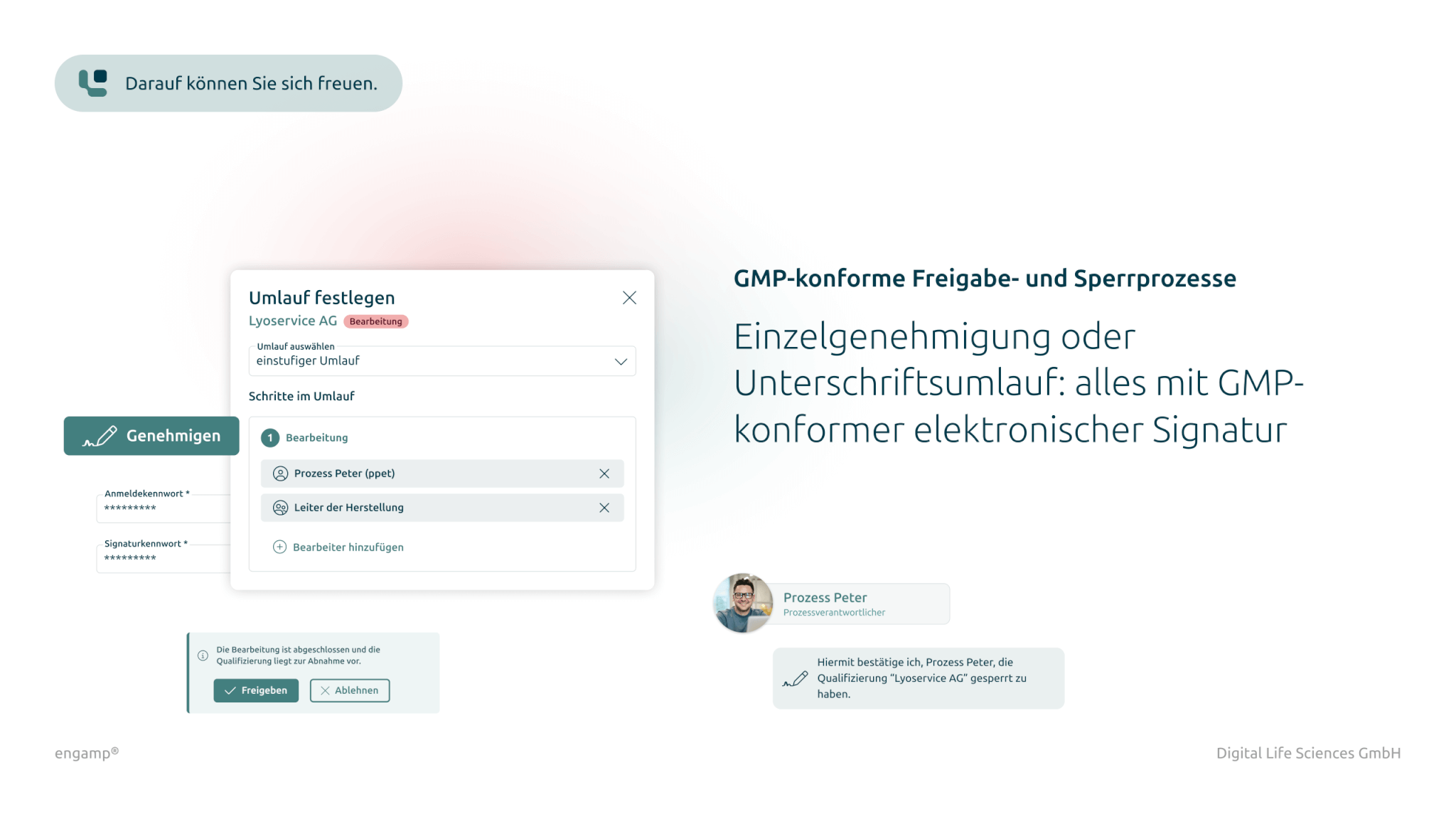
Documented and GMP-compliant release process
A GMP-compliant audit trail logs all relevant actions and ensures that approval decisions can be made on a reliable basis. This increases traceability and compliance in the qualification process.
06.
Automatic monitoring of certificates and qualifications
The software automatically monitors the validity of certificates and sends notifications when a renewal is necessary. This prevents interruptions in the supply chain and ensures high quality standards.
07.
Efficient audit management
The integrated planning tools optimise the entire audit process. Optimised planning and management of audits saves time and resources and ensures systematic and efficient implementation.
08.
Predefined templates for evaluation processes
Standardised templates for questionnaires, audit reports, and evaluations facilitate data collection, audit preparation, and reporting, thus increasing the efficiency of the entire supplier qualification process.
Advantages
Advantages of the supplier qualification software
Ensure the quality of your products and minimise risks in global supply chains through comprehensive supplier qualification. Start your success story today!
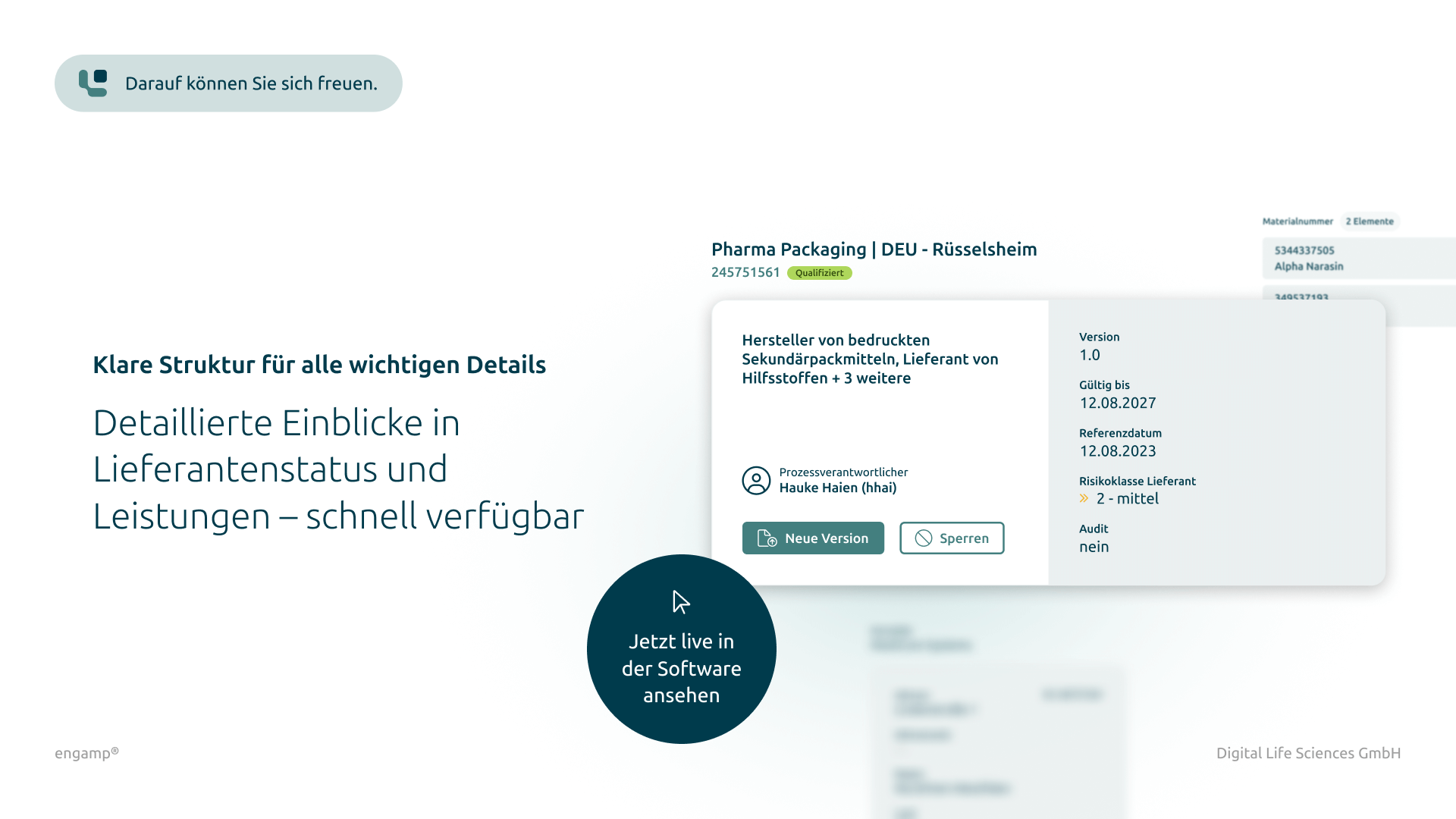
‘Single point of truth’ for the status of your suppliers
Supplier qualification serves as an essential source of information for transparently and reliably determining the status of suppliers from whom services and materials may be procured.
Representation of the supply chain based on material numbers
The visual representation of the supply chain from materials to the end product creates greater transparency for stakeholders and enables a differentiated view by target country.
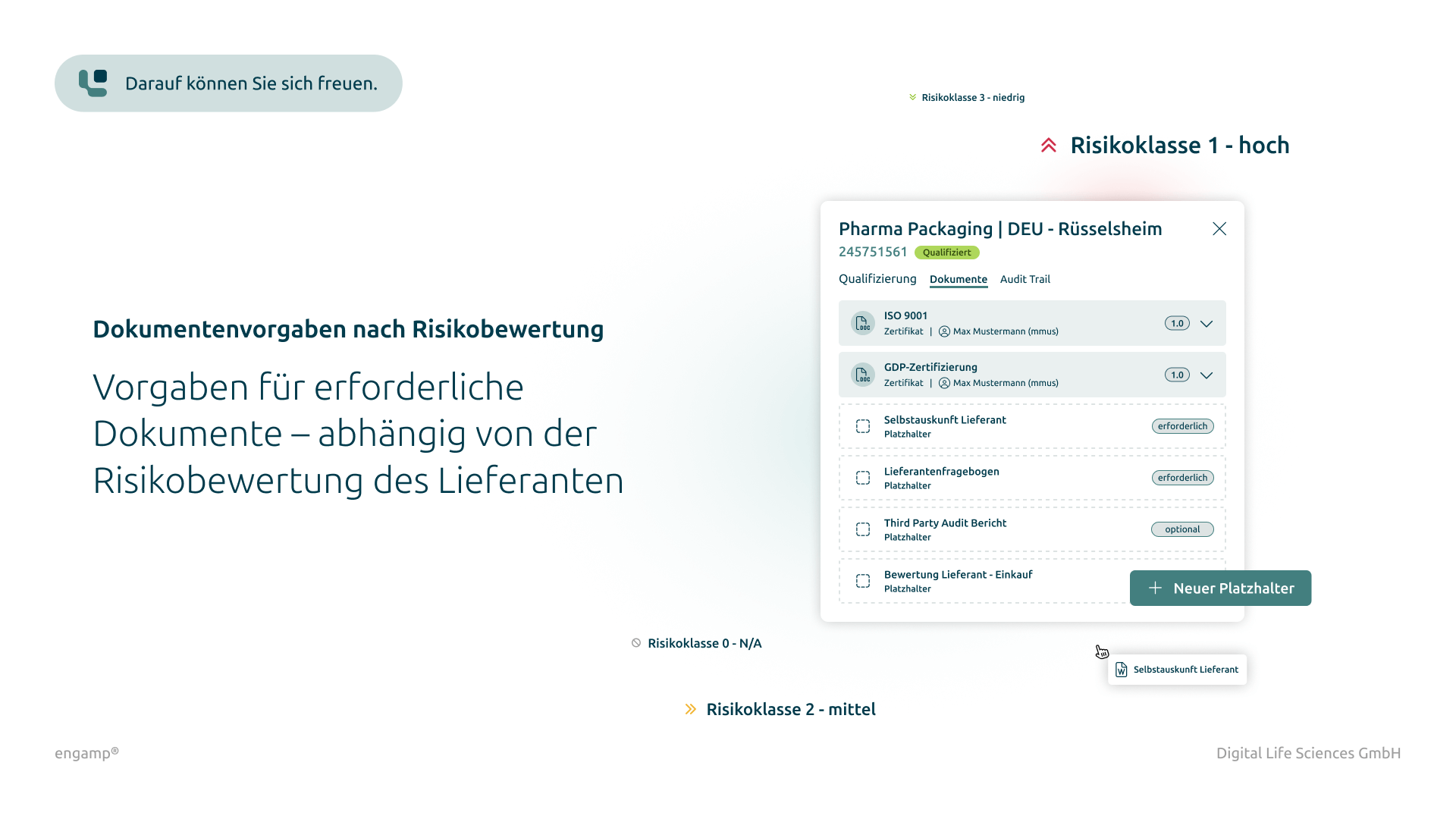
Risk-based definition of qualification measures
Defining the required documents based on your risk evaluation ensures compliance with regulatory requirements and avoids missing proofs.
Efficient planning and implementation of audits
Integrated tools for optimised planning and implementation of audits save time and resources and increase the overall efficiency of quality control processes.
Document dossier per qualification
A specific dossier with documents for each qualification documents the current status and enables direct access to proofs, certificates, contracts...
Documented release process
The GMP-compliant documentation of all relevant actions in the audit trail provides the person responsible for the release with a reliable basis for decision-making.
Monitoring of certificates and qualifications
Automatic monitoring and notification of the validity of certificates keep the certification status up to date, prevent interruptions in the supply chain due to expired certificates and help maintaining high-quality standards.
Templates for questionnaires, audit reports and evaluations
Predefined templates for different evaluation processes standardise data collection and reporting, facilitate audit preparation and conducting and increase efficiency.
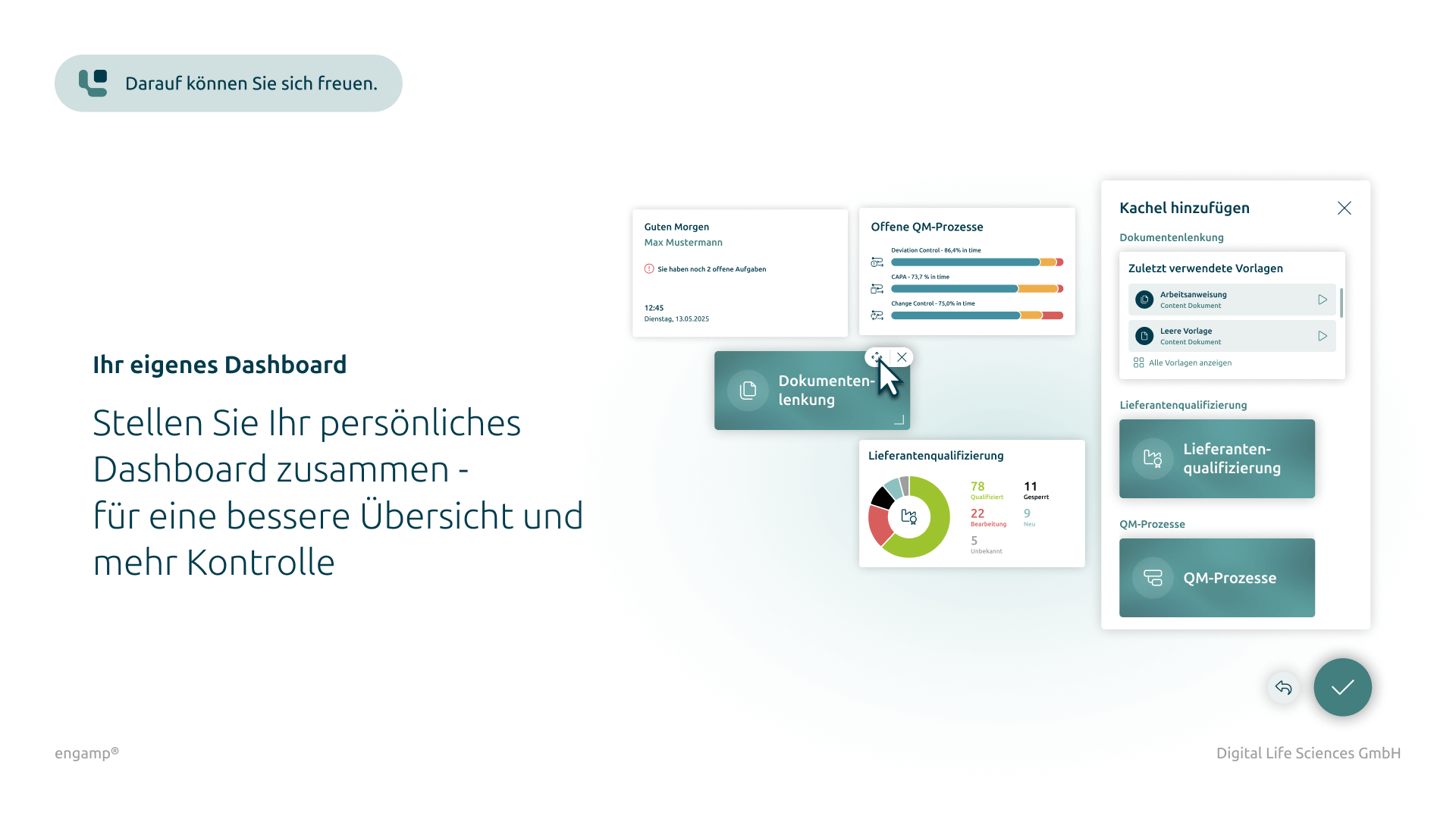
Features
Some features of supplier qualification
Ensure the quality of your products and minimise risks in global supply chains through comprehensive supplier qualification. Start your success story today!
‘Single point of truth’ for the status of your suppliers
Clear presentation of the supply chain based on material numbers in relation to the end products
Specification of the required documents according to the risk classes of your suppliers
Templates for questionnaires, audit reports, evaluations and much more.
Document dossier per qualification
Efficient planning and management of audits
Monitoring of certificates and qualifications
Features of supplier qualification
Important features of supplier qualification
The software engamp® | supplier qualification offers powerful functions for efficient and transparent supplier qualification.
‘Single point of truth’ for the status of your suppliers
All relevant information for evaluating and qualifying suppliers is bundled centrally, enabling well-founded decision-making.
Clear presentation of the supply chain based on material numbers
A clear visualisation of the supply chain from materials to end products creates transparency and supports the optimisation of procurement processes.
Document dossier per qualification
Each supplier qualification is documented with a specific dossier containing all relevant certificates, contracts and proofs.
Efficient planning and management of audits
Integrated tools enable optimised scheduling, implementation and documentation of audits, saving time and resources.
Specification of the required documents according to the risk classes of your suppliers
Based on a risk evaluation, the required documents are automatically defined so that no important proof is missing and regulatory requirements are always met.
Templates for questionnaires, audit reports, evaluations and much more.
Predefined templates facilitate the standardised collection of information, enable efficient audit execution and improve the comparability of supplier qualifications.
Monitoring the validity of certificates and qualifications
Automatic monitoring and notification of expiring certificates ensure continuous compliance with quality requirements.
Regulations for supplier qualification
Regulatory requirements in supplier qualification
The supplier qualification software was developed taking into account all relevant regulatory requirements in order to ensure a legally compliant and efficient evaluation and qualification of suppliers.
GMP Guideline, Chapter 1 & 5
Requirements for the quality management system (QMS) and supplier qualification in the pharmaceutical industry.
MDR 2017/745, Article 10 (9) d
Obligation of manufacturers of medical devices to carefully select and review their suppliers.
ISO 13485:2016, section 7.4.1
Requirements for supplier evaluation and qualification for companies in the medical technology sector.
FDA 21 CFR 820.50
US FDA requirements for supplier controls and procurement processes to ensure product quality.
Ordinance for the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients § 11
Regulatory requirements for the review and documentation of supplier qualification for pharmaceutical manufacturers in accordance with the German Ordinance for the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients (AMWHV).
ICH Q10, section 2.7
Requirements for a pharmaceutical quality system, including the control of suppliers and external partners.
Blog post supplier qualification
Development of the supplier qualification software
Read our blog post to find out how we designed and developed the software with some our customers.
-
Why supplier qualification is indispensable in the pharmaceutical industry
-
Why companies need to qualify their suppliers
-
Working together for greater efficiency — the cooperation with MEDICE & Rottendorf Pharma
-
The pilot project — digitalisation as the key to efficient supplier qualification
-
The development process — from the initial vision to implementation
-
Digitalisation of supplier qualification – a holistic solution for the pharmaceutical industry
Testimonials
Inspiring customer opinions on the supplier evaluation software
Discover how companies in your industry have been transformed by using our software solutions. Read authentic success stories that testify to sustainable growth and innovative approaches.
Years ago, we used to track the status of documents such as work instructions, employee qualification records and batch reports using an Access database or on paper. However, it was tedious to keep the documents up to date and to manage them in an audit-compliant manner. In 2012, we switched to digital document control for Digital Life Sciences, which was a quantum leap. Today we can create, find, edit and agree upon documents much faster. This saves working time and costs.

Christine Strubel
Head of Management Systems Quality & EHS (SCHOTT AG)
FAQ about supplier qualification
Frequently asked questions (FAQs) about supplier qualification
This guide will help you learn more about our services and their features
Expand FAQs
Supplier qualification involves the evaluation and selection of suppliers to ensure that they meet a company's quality and compliance requirements. The process includes:
- Risk evaluation
- Audits and document review
- Regulatory compliance check
- Ongoing monitoring of supplier performance
The aim is to guarantee a reliable supply chain and ensure that materials and services meet the specified quality standards.
Supplier evaluation is an integral part of quality management. In regulated sectors, such as the life science industry, it is particularly important in order to comply with legal requirements.
Step 1: Needs analysis & recording of requirements
- Definition of requirements for raw materials, packaging materials or services
- Determination of sources of supply and responsibilities (e.g. intermediaries)
Step 2: Supplier selection
- Research potential suppliers
- Preselection based on criteria such as quality, price, delivery reliability, certifications
Step 3: Risk evaluation
- Classification of suppliers into risk classes
- Determination of the necessary GMP requirements
Step 4: Qualification measures
- Requesting and reviewing documents such as certificates or references
- On-site audits or self-evaluation questionnaires
- Quality agreements on storage, transportation and deviations
Step 5: Delivery & Monitoring
- Collection of analysis data and audit results
- Monitoring of official inspection results
Step 6: Requalification
- Regular re-evaluation of suppliers to ensure compliance
Step 7: Evaluation of changes
- Changes at the supplier are documented and evaluated in the Change Control process
This structured process ensures that all necessary qualifications are met and that quality is consistently guaranteed throughout the supply chain.
Yes, requalification is required if:
- The validity of a qualification expires
- Changes at the supplier occur (e.g. new production sites, certificate changes)
The qualification process is then carried out again to ensure that the supplier continues to meet the requirements.
Supplier evaluation in accordance with Good Distribution Practice (GDP) and Good Manufacturing Practice (GMP) requires:
- Systematic review of the supplier's compliance
- Collection and analysis of relevant quality data
- Regular audits & Certificate inspections
This ensures continuous supplier development and quality assurance.
Data is essential for reviewing GDP and GMP compliance. By systematically recording, analysing and documenting supplier data, it is possible to:
- Recognise missing proof at an early stage
- Compare suppliers
- Optimise processes and ensure compliance
Yes, in addition to the standardised supplier types, individual material numbers can be assigned to a qualification.
The period of validity of a qualification is calculated based on the defined risk class.
Yes, the software provides a transparent representation of the supply chain by:
- Linking material numbers, products and destination countries
- Making the use of raw materials through to the end product traceable
Yes, the document dossier is a central component of the software. Depending on the risk class, checklists are automatically created with the required documents, including
- Certificates
- Contracts
- Audit reports
These documents can be uploaded, versioned and controlled.
Yes, the software supports efficient audit planning:
- Scheduling and audit teams
- Self-conducted audits or third-party audits
- Creation and management of audit reports
Contact supplier qualification
Are you ready to optimise your supplier processes?
Turn potential into success – use our pioneering QM cloud solutions!
Would you like us to call you back?
We will be happy to call you back. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.
Are you interested in a presentation?
We are happy to offer you a presentation free of charge. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.