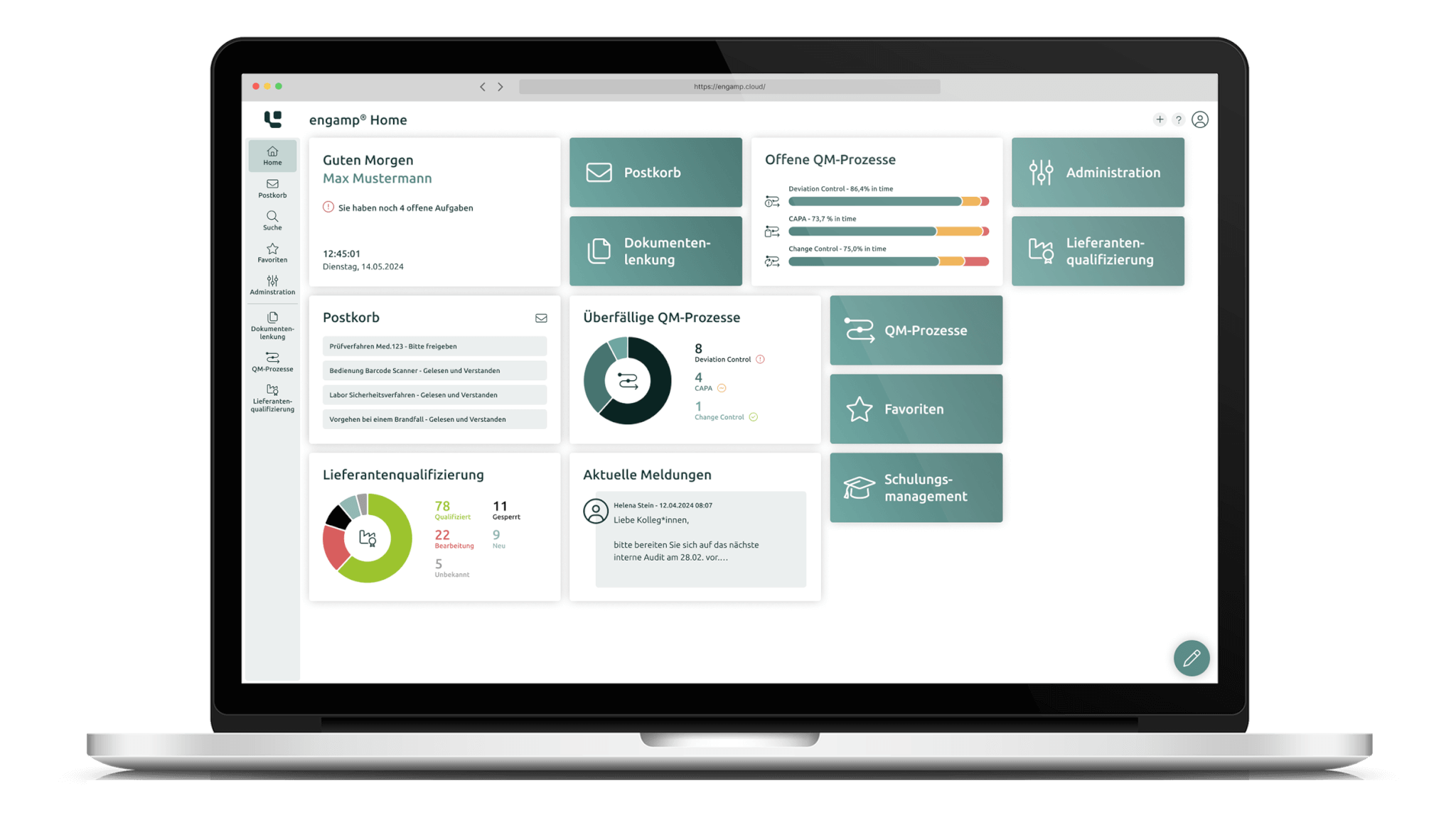
Document control software
Manage your documents digitally
Whether SOPs, process descriptions, test specifications or other document types – with the document control software you can create, edit, and sign all these documents digitally. Start your digital transformation today!
- Digital management
- Compliant with standards
- GxP-compliant signature
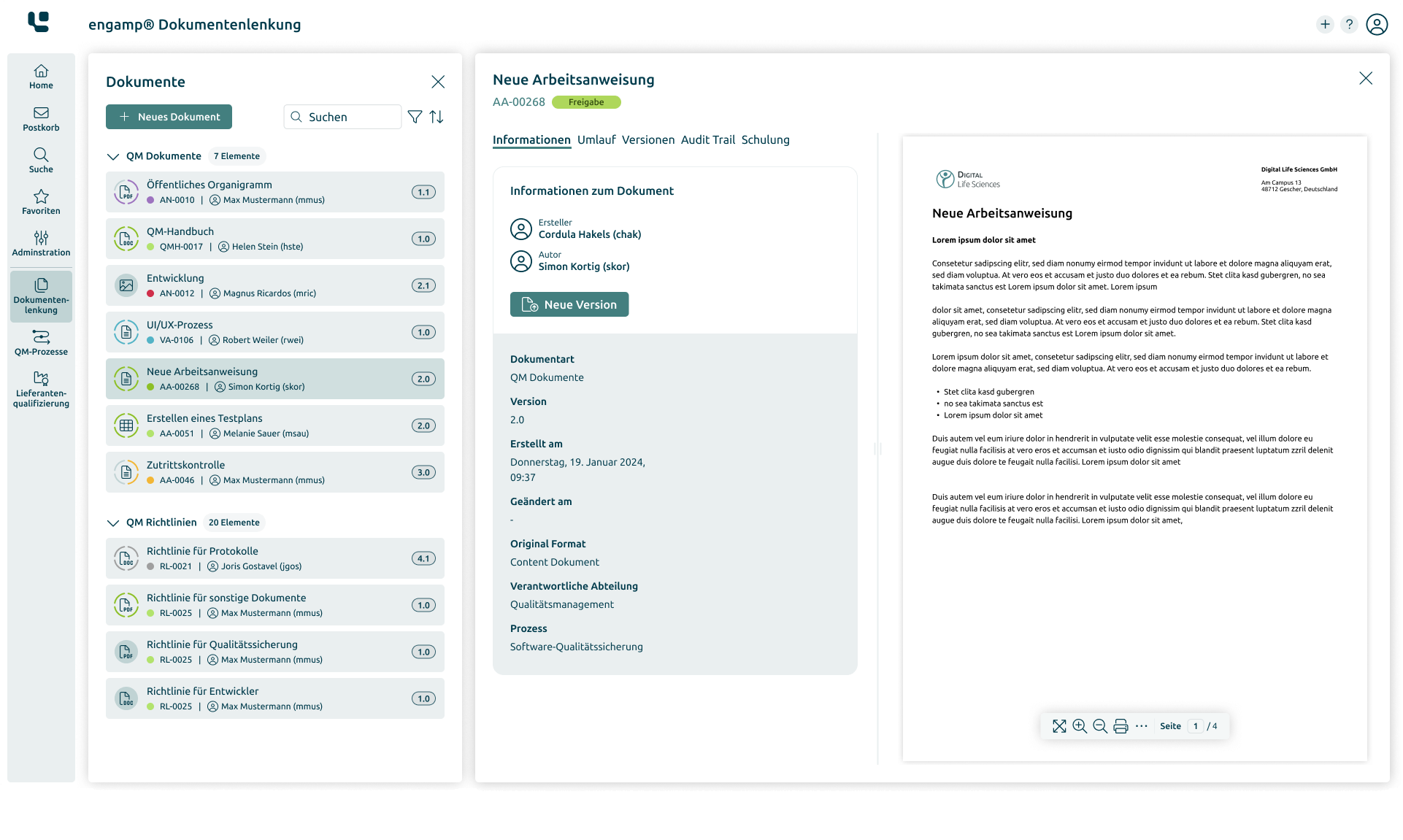
How does document control provide support?
Optimised document control
"engamp® | document control" offers a powerful solution for the efficient control of business-critical documents and fulfills the highest regulatory requirements. It enables the creation, revision, and digital signing of SOPs, process instructions, test instructions, operational guidelines, manufacturing documents, and contracts. The system flexibly adapts single and multi-stage approval processes and automatically delegates periodic review tasks. Reminder and escalation management prevents deadlines from being exceeded, while audit trails ensure compliance with regulations.
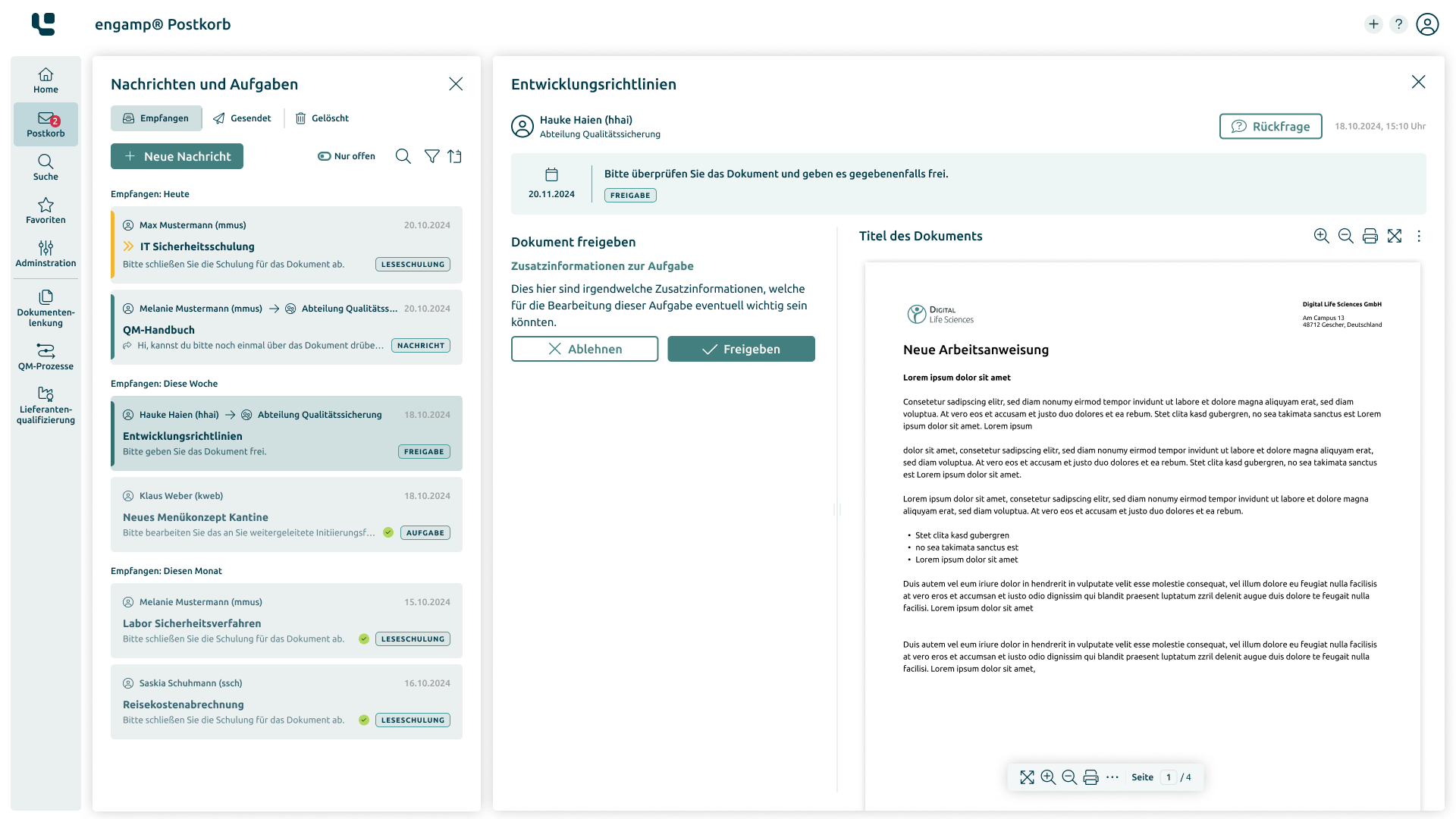
Advantages of document control
Increase your efficiency with digital document control
In an increasingly digitalised business world, well thought-out and efficient document management is essential. The engamp® | document control software solution enables companies to optimise their documentation processes, meet regulatory requirements, and save valuable resources at the same time.
01.
Structured digital document management
Maintain an overview of all specification documents, including work and process instructions, test specifications, and hygiene plans. Forms, contracts, and other business-related documents can also be stored and managed securely and centrally.
02.
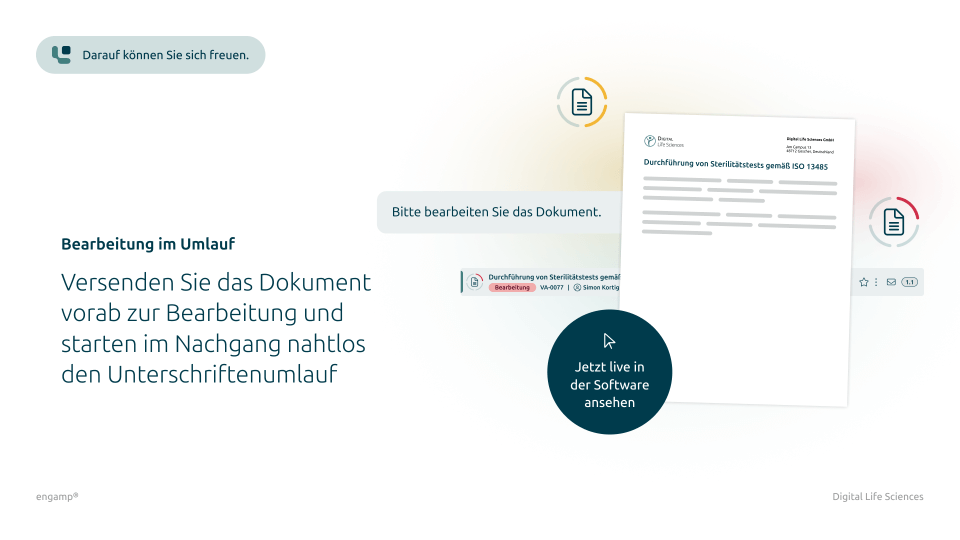
Automated circulations at the touch of a button
Manual distribution of documents is a thing of the past. With just one click, documents can be distributed in an audit-proof manner and the entire signature process can be handled digitally. This leaves more time for strategic tasks and the core business.
03.
Accelerated process runtimes
Optimise your workflows: The creation, revision, release and distribution of documents takes place with significantly reduced processing times. This makes processes more efficient and avoids bottlenecks.
04.
Sustainability and resource conservation
Reduce unnecessary printouts and cut paper consumption in your company. This does not only protect the environment, but also contributes to cost savings and a more efficient way of working.
05.
Central information platform for maximum transparency
You have a powerful central information platform at your disposal that enables audit-proof filing and quick access to relevant documents.
06.
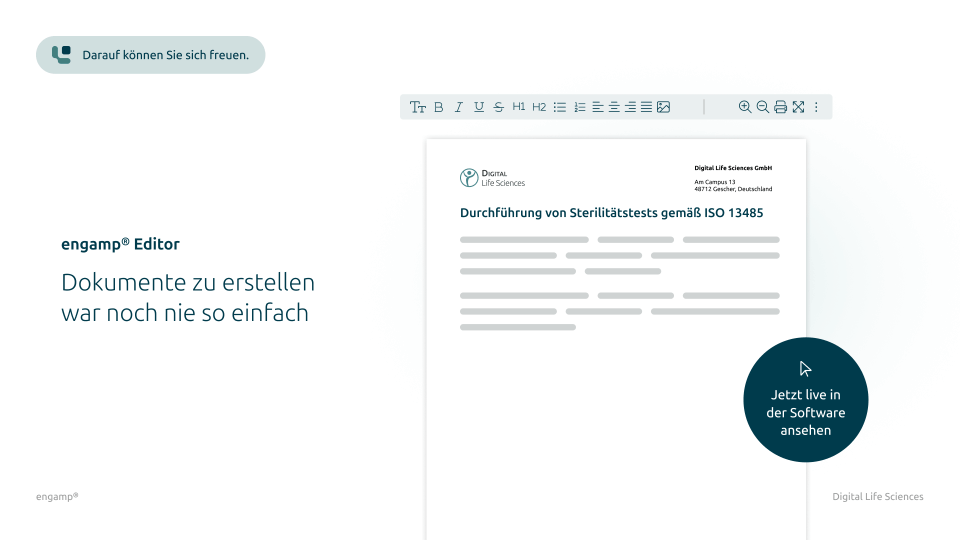
Direct document creation without media disruptions
With the integrated editor, you can create and edit your documents directly in the system – without any external programs. This eliminates media disruptions and avoids potential data integrity risks.
07.
Maximum data security
Sensitive company information deserves maximum protection. Intelligent authorisation structures and encrypted filing systems ensure that only authorised persons can access relevant documents. At the same time, potential threats from unauthorised access or data loss are minimised.
08.
Individually customisable configuration
Every company has specific requirements. The software can be flexibly adapted to individual requirements to enable seamless integration into existing processes. Intuitive operation also ensures that new users are quickly familiarised with the system.
09.
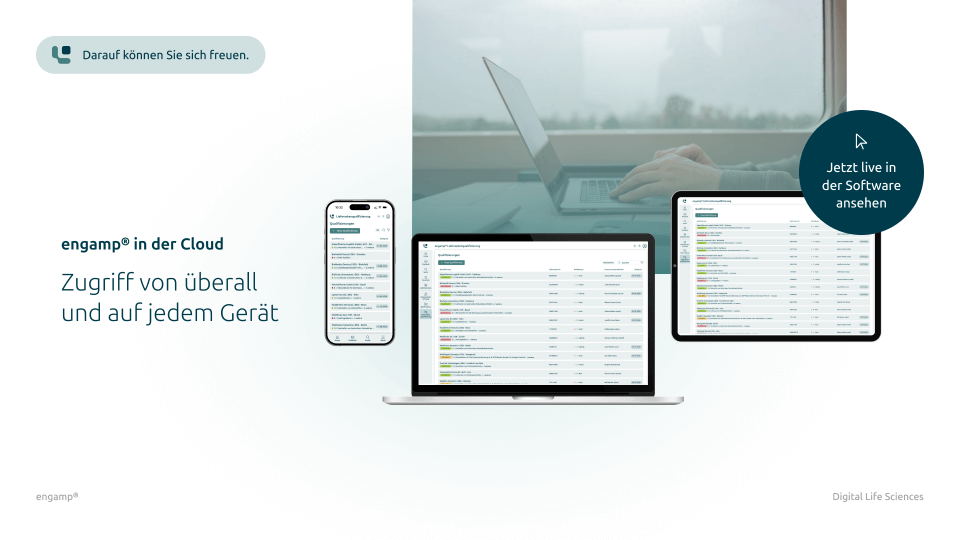
Flexible access – anytime and anywhere
Whether you're in the office, working from home or on the move – with a cloud-based solution, you can access your documents at any time and from anywhere to stay productive.
What does digital document control look like?
Get a first insight
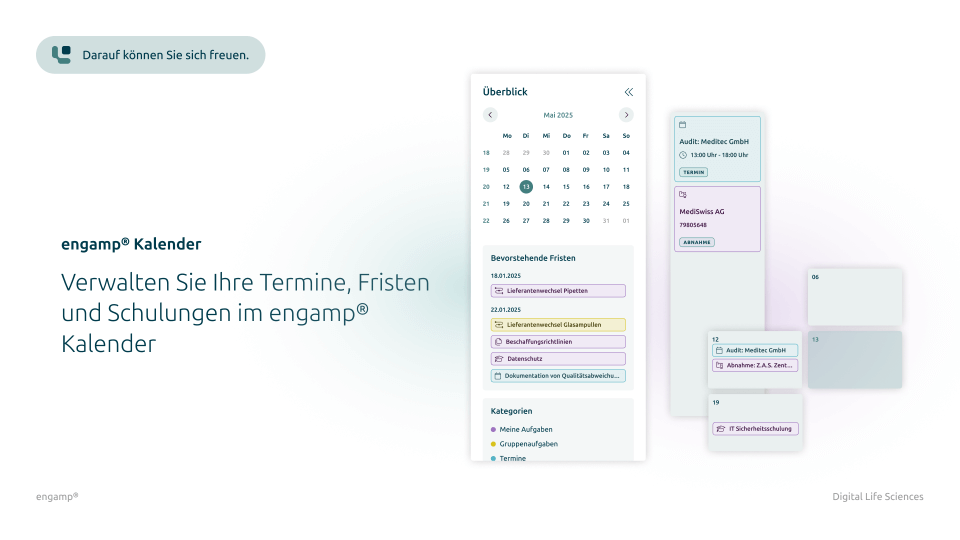
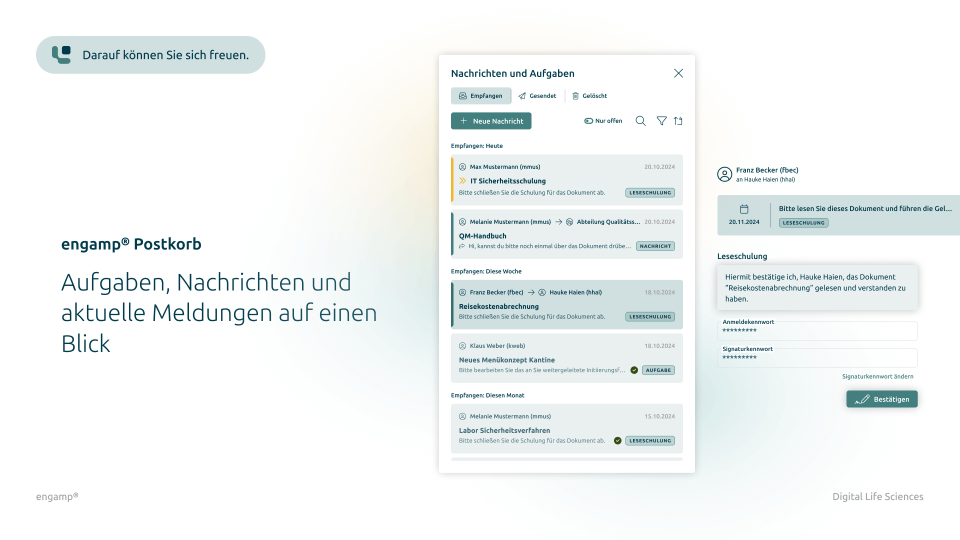
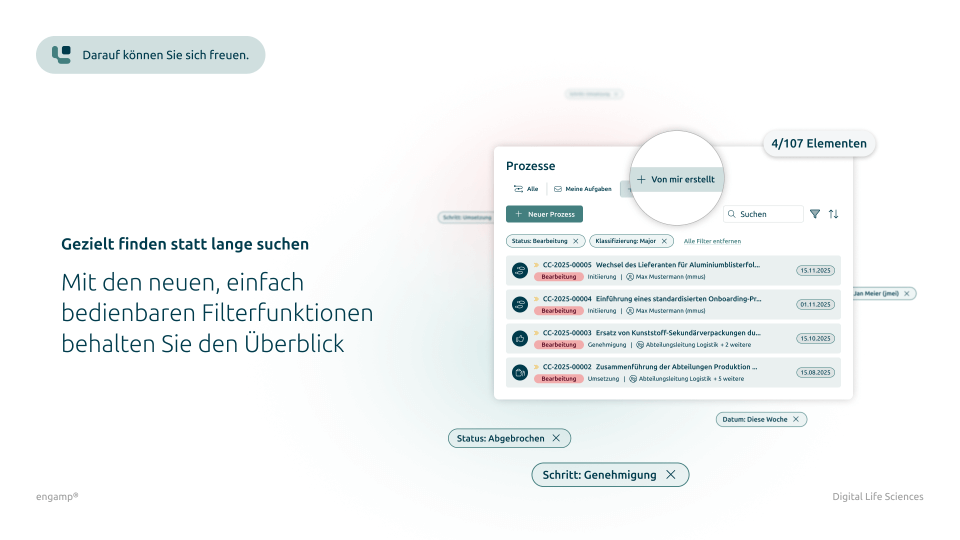

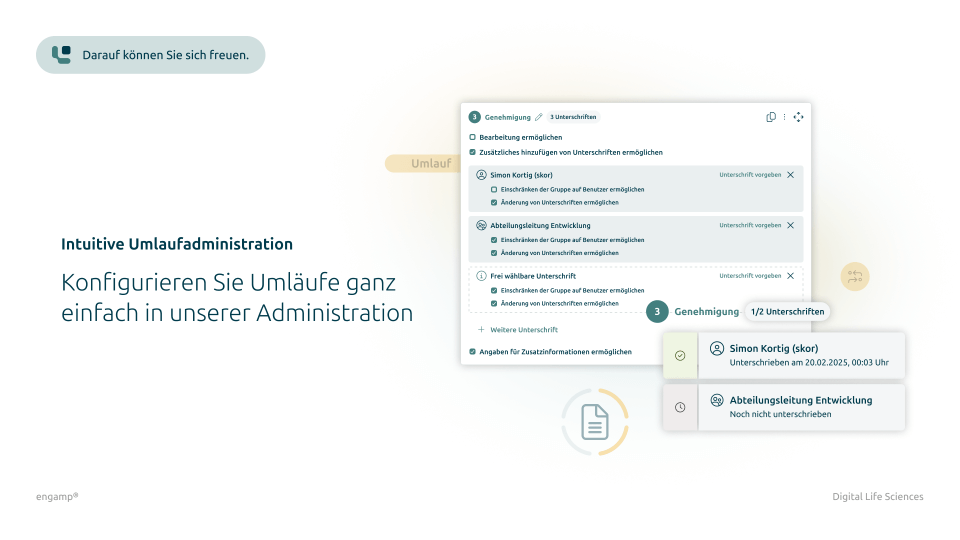
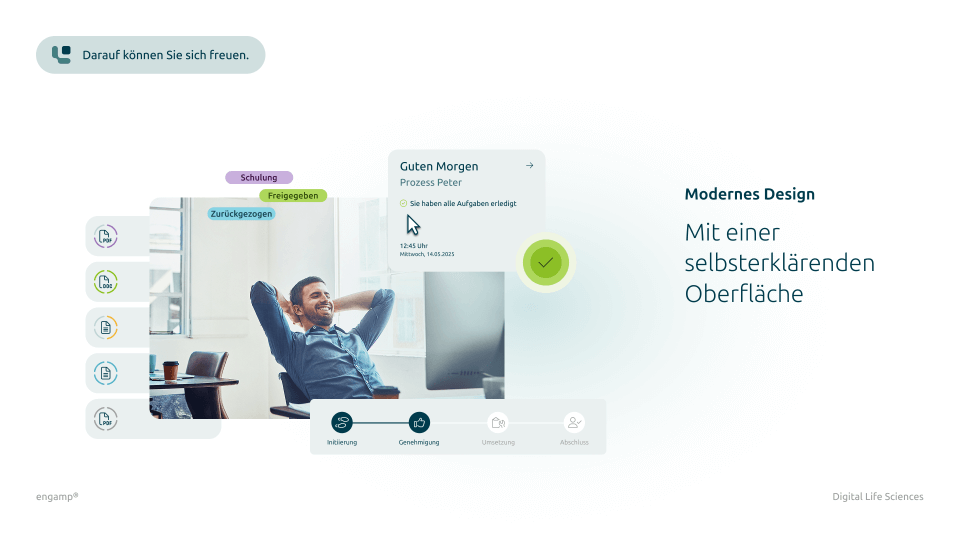
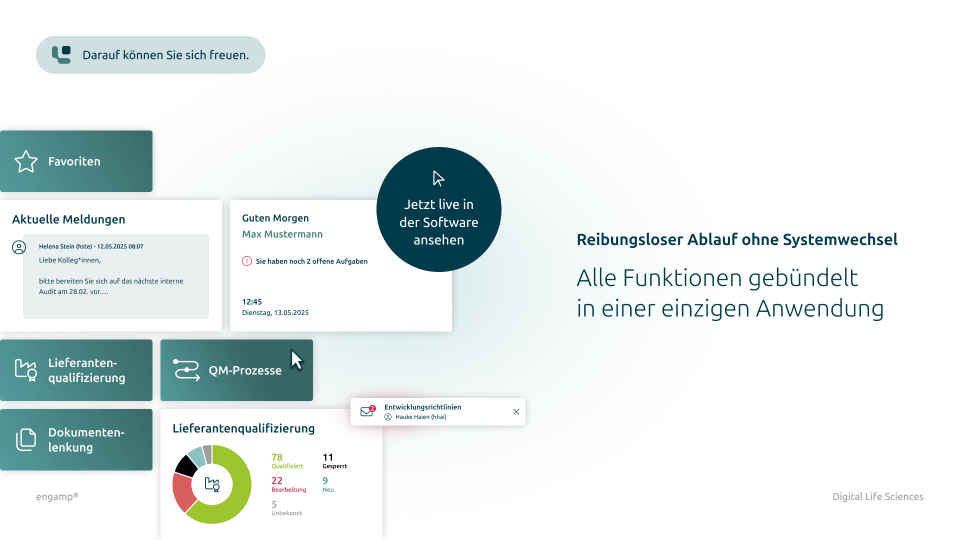
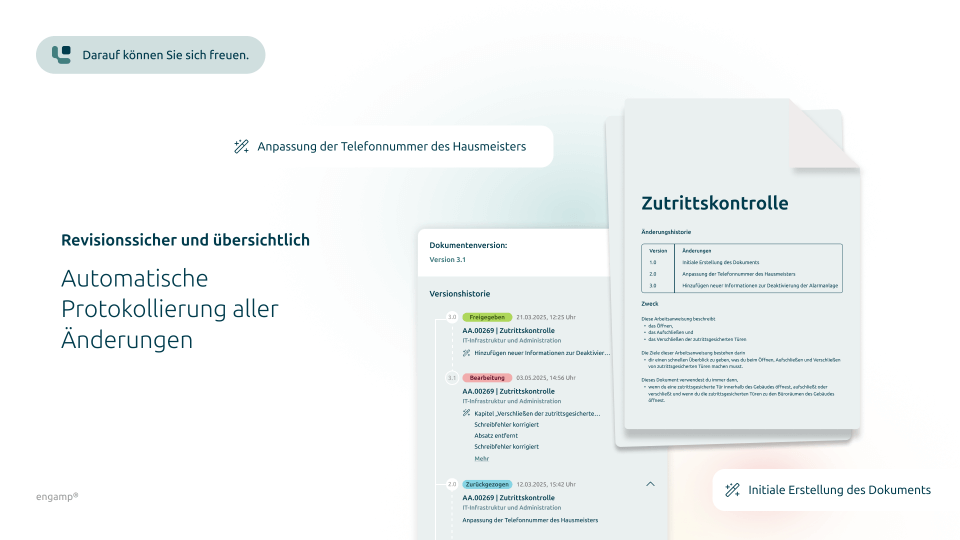
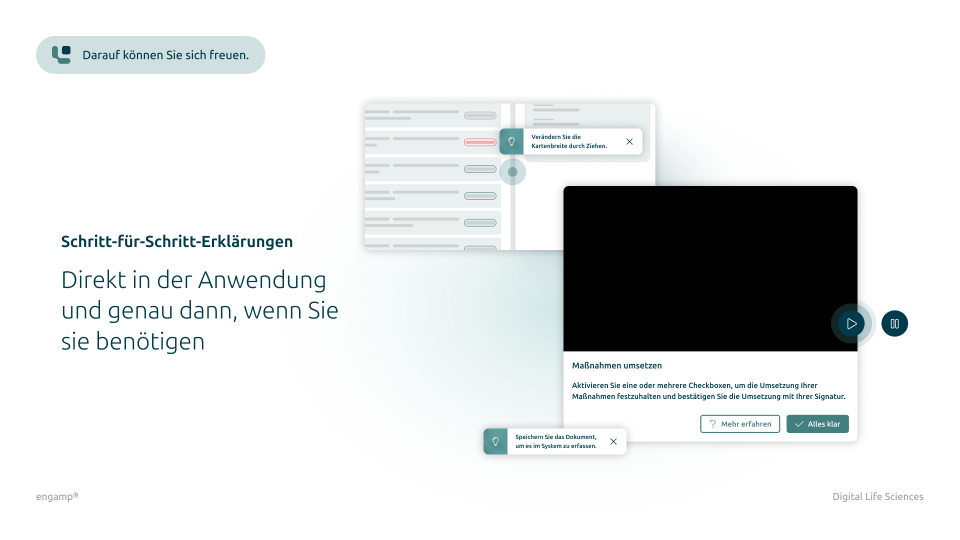
Features
Some features of the supplier qualification
Ensure the quality of your products and minimise risks in global supply chains through comprehensive supplier qualification. Start your success story today!
'Single point of truth' about the status of your suppliers
Clear presentation of the supply chain based on material numbers in relation to the end products
Specification of the required documents according to the risk classes of your suppliers
Templates for questionnaires, audit reports, evaluations and much more.
Document dossier per qualification
Efficient planning and management of audits
Monitoring of certificates and qualifications
Features of document control
Main functions of document control
Our document control software offers a wide range of powerful functions that enable efficient, transparent, and compliant management of your documents.
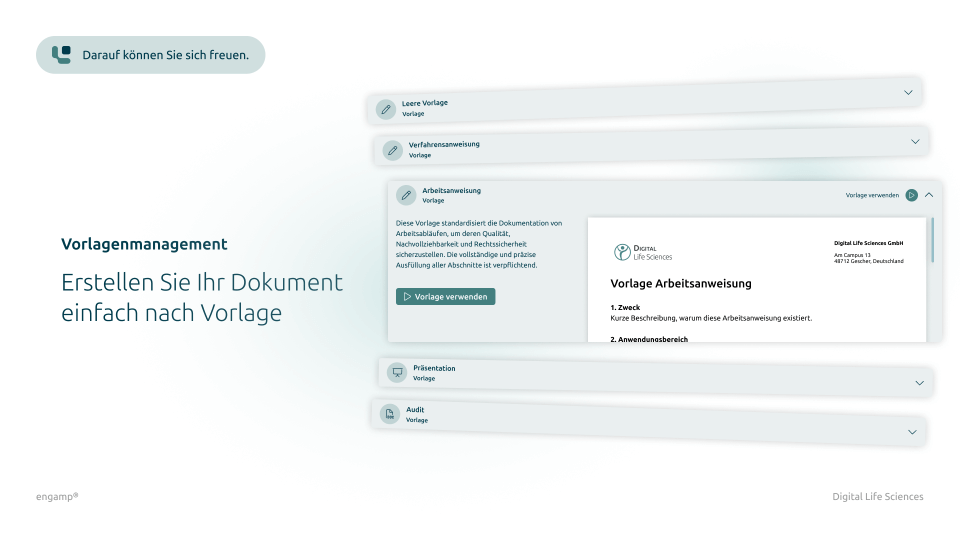
Creation and management of controlled documents
✔ Creation of controlled documents directly in the system based on previously approved templates ✔ Templates themselves can be managed and versioned as controlled documents ✔ Structured management of specification documents, work instructions, and forms
Electronic signature & traceability
✔ Digital signature using an integrated, GxP-compliant signature – fully software-based and secure ✔ Logged workflows for maximum traceability ✔ Automated "Periodic review" function for the timely review of documents
Advanced search and comparison features
✔ Full-text search across the entire document content and associated metadata ✔ Direct comparison of different document versions with display of changes
Security & protection mechanisms
✔ Individually customisable, dynamic forced watermark for increased security ✔ Controlled and logged printout to prevent unauthorised distribution
Efficient reporting and archiving functions
✔ Convenient creation and management of report lists ✔ Integrated audit-proof archiving with the ECM system d.velop documents (formerly d.3ecm)
Flexible use & user-friendliness
✔ Intuitive web front-end for maximum user-friendliness ✔ Flexible access options – can be used at any time and from any location
Regulations
Regulatory requirements for controlled documents
The management of controlled documents is subject to a large number of regulatory requirements that ensure that documentation processes are carried out in a traceable, secure manner, and in compliance with international standards. Some of the most important regulations that must be observed in document control are listed below:
ISO 9001:2015, Chapter 7.5
✔ Requirements for the documentation of information as part of a quality management system (QMS). ✔ Ensuring the availability, protection, control, and updating of documented information.
ISO 13485:2016, Chapter 4
✔ Specific requirements for document control for manufacturers of medical products. ✔ Requires audit-proof management of quality documents.
FDA 21 CFR Part 11
✔ Regulations of the US Food and Drug Administration (FDA) for the use of electronic records and signatures. ✔ Requirements for authenticity, integrity, availability, and traceability of electronic documents.
EU-GMP guideline, Annex 11
✔ Requirements for computerised systems to ensure data integrity in the pharmaceutical industry. ✔ Refers to software validation, audit trails, and electronic signatures.
WHO Guidelines on Good Practices in Data and Record Management
✔ World Health Organization guidelines for ensuring data integrity, traceability, and authenticity in regulated industries.
EFG Votum V11002: Requirements regarding the electronic data storage
✔ Defines standards for the long-term archiving of electronic data, taking into account security, integrity, and access protection.
EFG Votum V11003: Requirements regarding electronic signatures and initials
✔ Specifies the criteria that electronic signatures must meet in order to be legally binding and tamper-proof.
Regulations for document control
Regulatory requirements for controlled documents
The management of controlled documents is subject to a large number of regulatory requirements that ensure that documentation processes are carried out in a traceable, secure manner, and in compliance with international standards. Some of the most important regulations that must be observed in document control are listed below:
ISO 9001:2015, Chapter 7.5
✔ Requirements for the documentation of information as part of a quality management system (QMS). ✔ Ensuring the availability, protection, control, and updating of documented information.
ISO 13485:2016, Chapter 4
✔ Specific requirements for document control for manufacturers of medical products. ✔ Requires audit-proof management of quality documents.
FDA 21 CFR Part 11
✔ Regulations of the US Food and Drug Administration (FDA) for the use of electronic records and signatures. ✔ Requirements for authenticity, integrity, availability, and traceability of electronic documents.
EU-GMP guideline, Annex 11
✔ Requirements for computerised systems to ensure data integrity in the pharmaceutical industry. ✔ Refers to software validation, audit trails, and electronic signatures.
WHO Guidelines on Good Practices in Data and Record Management
✔ World Health Organization guidelines for ensuring data integrity, traceability, and authenticity in regulated industries.
EFG Votum V11002: Requirements regarding the electronic data storage
✔ Defines standards for the long-term archiving of electronic data, taking into account security, integrity, and access protection.
EFG Votum V11003: Requirements regarding electronic signatures and initials
✔ Specifies the criteria that electronic signatures must meet in order to be legally binding and tamper-proof.
Which companies rely on digital document control?
Inspiring customer opinions on digital document control
Discover how companies in your industry have been transformed by using our software solutions. Read authentic success stories that testify to sustainable growth and innovative approaches.
Years ago, we still managed the status of our documents, including work instructions, employees' proofs of qualification and batch reports, manually – either with an Access database or even on paper. The effort involved in keeping these documents up to date and archiving them in an audit-proof manner was enormous. In 2012, we switched to digital document management from Digital Life Sciences – a real quantum leap for our company. Today, we can create documents much faster, find them quickly, edit them efficiently and release them in an audit-proof manner. This has not only optimised our work processes, but also saves us considerable time and money.

Björn Boxhammer
Head of Quality Assurance & IT (Homöopathisches Laboratorium Alexander Pflüger GmbH & Co. KG)
FAQ on document control
Frequently asked questions (FAQ's) about document control
This guide will help you learn more about our services and their features
Expand FAQ's
Yes, the dls | eQMS supports the management of quality management documents in three main categories:
- QM manual: Contains generally applicable documents such as company guidelines and refers to detailed process specifications.
- Process instructions: Define workflows and processes in the company.
- Work instructions: Describe specific activities and workflows for employees.
These categories form the hierarchy levels of the QM documentation and enable structured management.
Yes, the document circulation can be flexibly adapted to company-specific requirements. This includes, for example, the processes of review, approval, release, training and setting effective. Each circulation can be individually linked to a document template so that different document types run through specific workflows.
Yes, employees and roles can be specifically defined for each step in the document circulation process. Minimum or maximum numbers of participants can be defined and certain employees or profiles can be designated as mandatory participants.
Yes, implementation is usually automated after completion of the planned training phase. Alternatively, you can also carry out the activation manually if a different release is required.
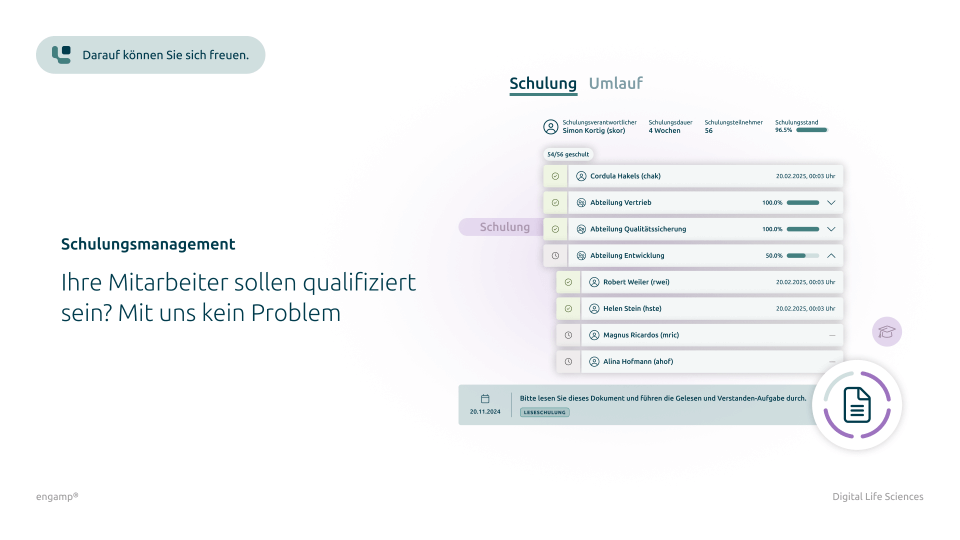
Yes, it is possible to configure a periodic review. The approver receives automated deadline proposals (e.g. two years after the document has become effectiv) and a corresponding review task is sent in advance. This review can be delegated to different persons or roles (e.g. reviewer, approver, releaser).
A " binding for list" can be created via a document distribution list. Once a document has been released, all relevant employees receive a task for Read and understood training. In addition, the document distribution list can be expanded so that employees can forward the document to other people.
Digital document control ensures that all documents are created, managed, archived, and disposed of in a structured manner. By using a document management system (DMS) such as dls | eQMS, the entire lifecycle from creation to archiving is optimised.
Systematic control ensures that all changes are documented in a traceable manner. The DMS enables the complete tracking of modifications and thus contributes to quality assurance and consistency.
An eQMS such as dls | eQMS ensures centralised storage and structured management of documents. This facilitates secure access, versioning, and legally compliant archiving of business-relevant information.
Yes, thanks to the integrated versioning and access control, several authorised users can work on one document without losing any information.
Yes, each document can be given a unique number. Hierarchical numbering is possible, which ensures, for example, that a subordinate SOP is only released once the higher-level document has already been approved.
Further applicable documents or attachments can be assigned to each controlled document. They appear in the document properties and in the preview. It is also possible to configure that authors are notified when a new version of a further applicable document is released.
Yes, printouts of controlled documents are logged. For controlled printouts, the employee must specify the extent to which the document is to be printed and for what reason. This information is recorded in the audit trail.
If the printout is later returned or destroyed, this can be recorded in the system using the function "Printout confirmation".
All actions requiring a signature in document circulation are carried out using a GxP-compliant electronic signature. This fulfills the requirements of the FDA (21 CFR Part 11) and the EU GMP guidelines and replaces the handwritten signature.
Each signature is documented in the audit trail and can be output as an additional page with the document. This ensures a legally binding and traceable signature.
Yes, the software uses major and minor versioning. If a document is rejected in an approval step, a new processing version can be created and the circulation can be restarted.
All versions are retained and can be viewed by authorised users. A direct comparison of two versions is also possible.
A DMS, such as the one contained in the dls | eQMS, offers numerous advantages:
✔ Electronic archiving for secure and legally compliant document storage
✔ Transparent workflows for efficient document management
✔ Automatic versioning and traceability
✔ Central document management for easy access and better organisation
Structured document management ensures that all relevant documents are centrally available. This promotes employee qualification, ensures uniform working methods and contributes to the optimisation of internal processes.
Document control is of central importance in risk management, as it ensures that all relevant information is systematically controlled and centrally managed. A clearly structured approach ensures that risks are precisely documented and important information is always available. This organised information management helps to identify potential risks at an early stage and take appropriate measures, thereby optimising risk management as a whole.
Contact document control
Are you ready to optimise your document processes?
Turn potential into success – Use our pioneering QM cloud solutions!
Would you like us to call you back?
We will be happy to call you back. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.
Are you interested in a presentation?
We are happy to offer you a presentation free of charge. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.