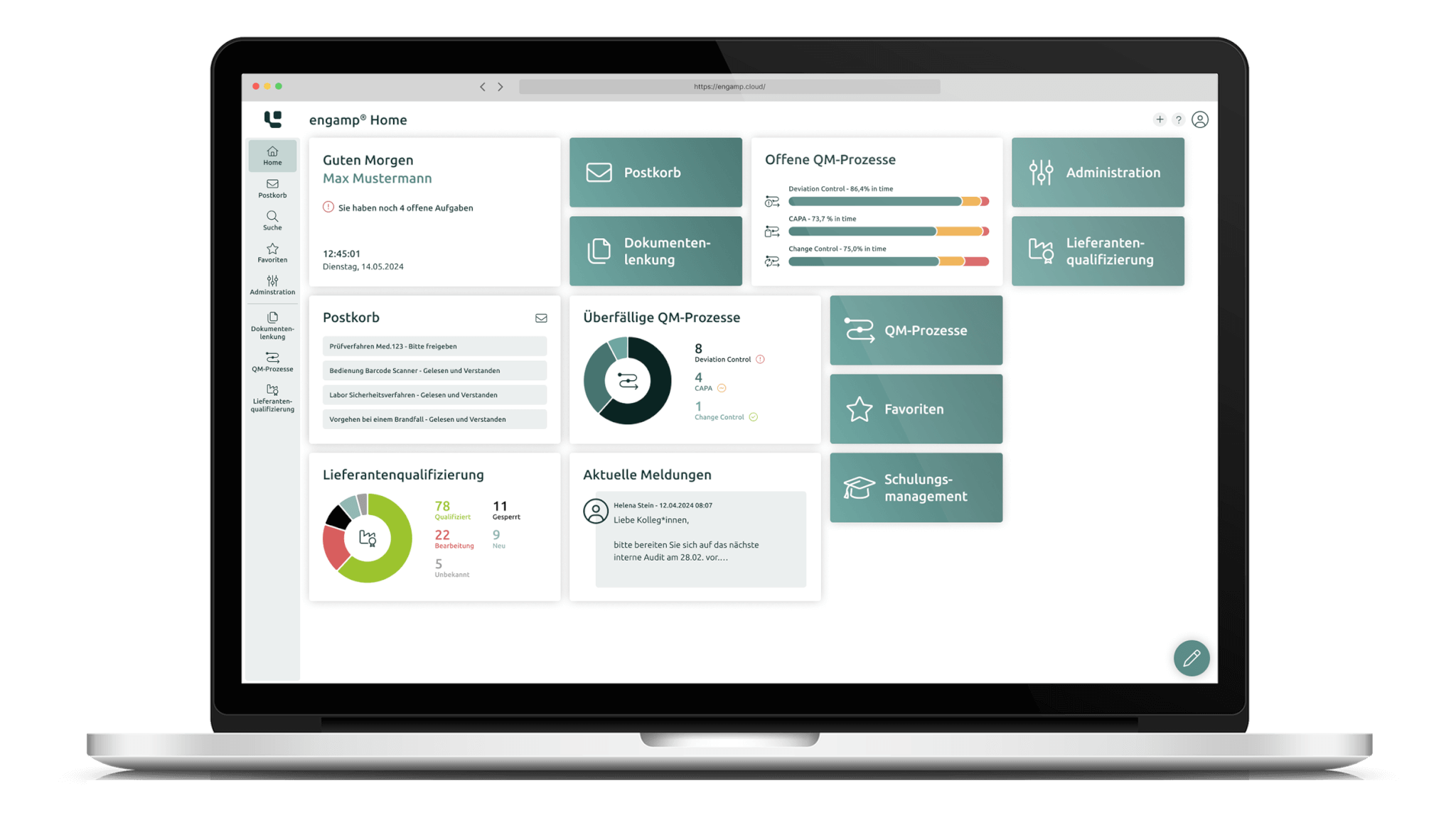
Digitally transforming QM – intelligently controlling processes with engamp®
Set new standards in quality management – with engamp® from Digital Life Sciences. Discover how innovative software solutions guarantee the secure and legally compliant archiving of your documents and benefit from efficient control of your quality management processes.
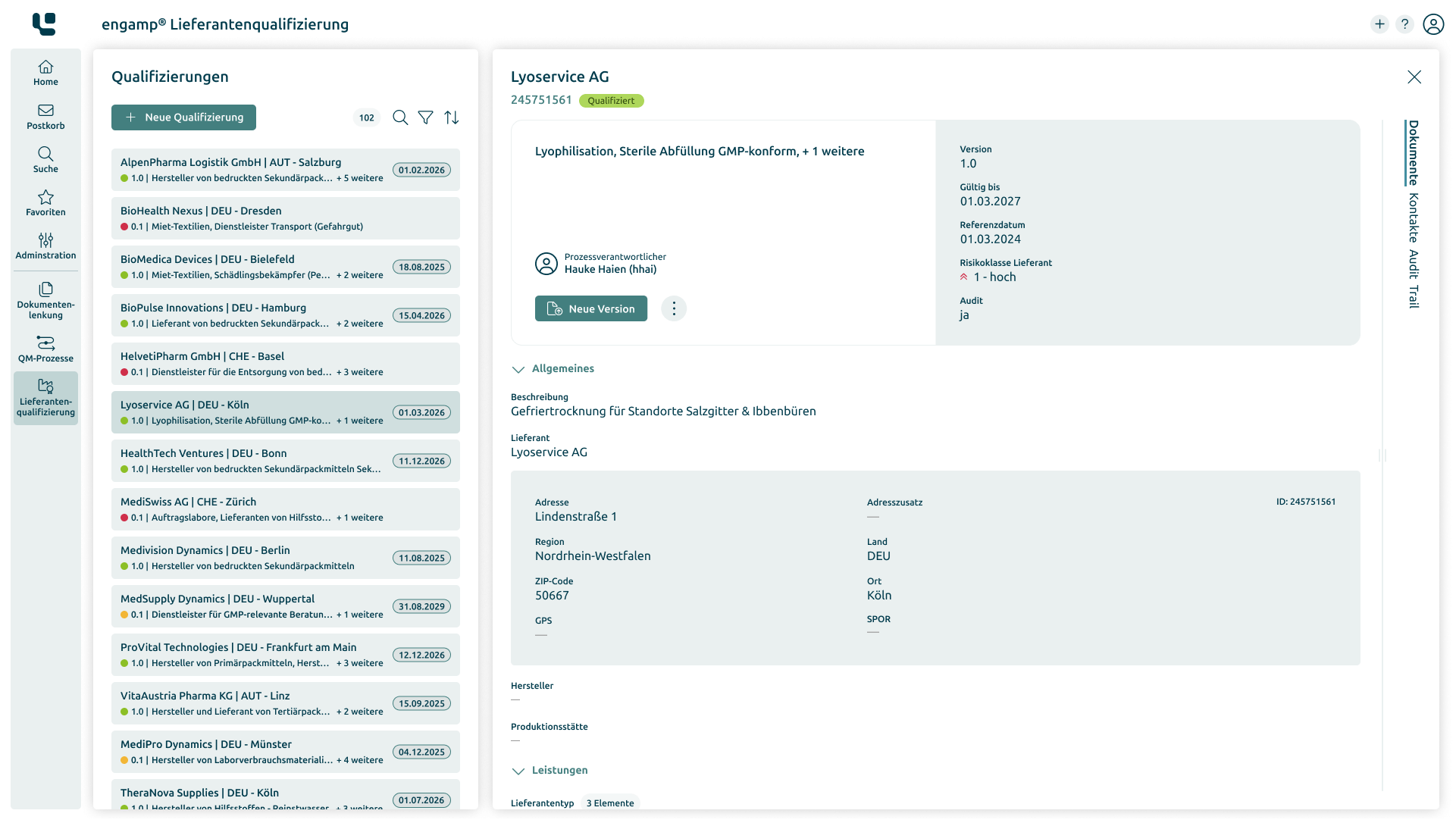
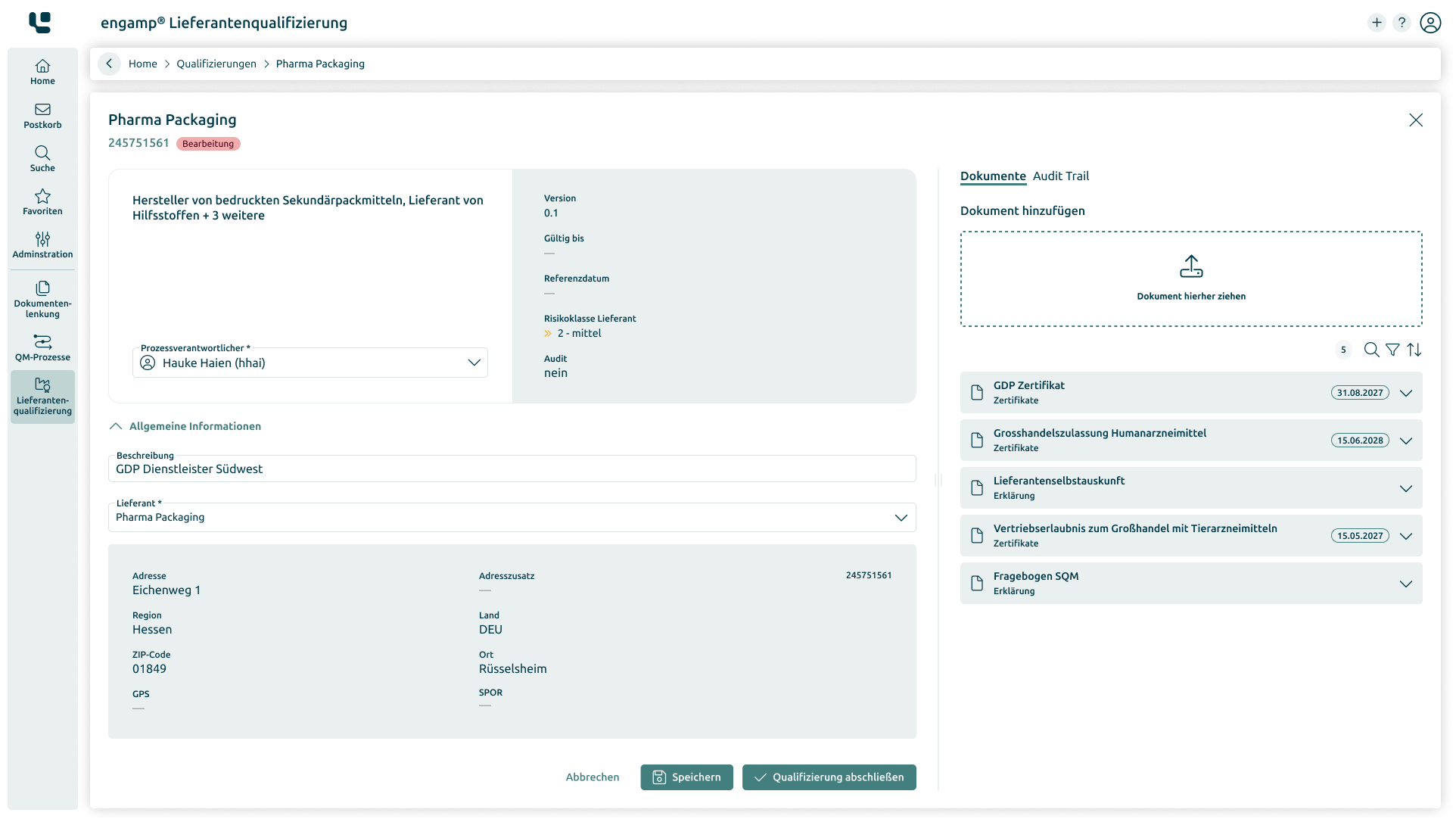
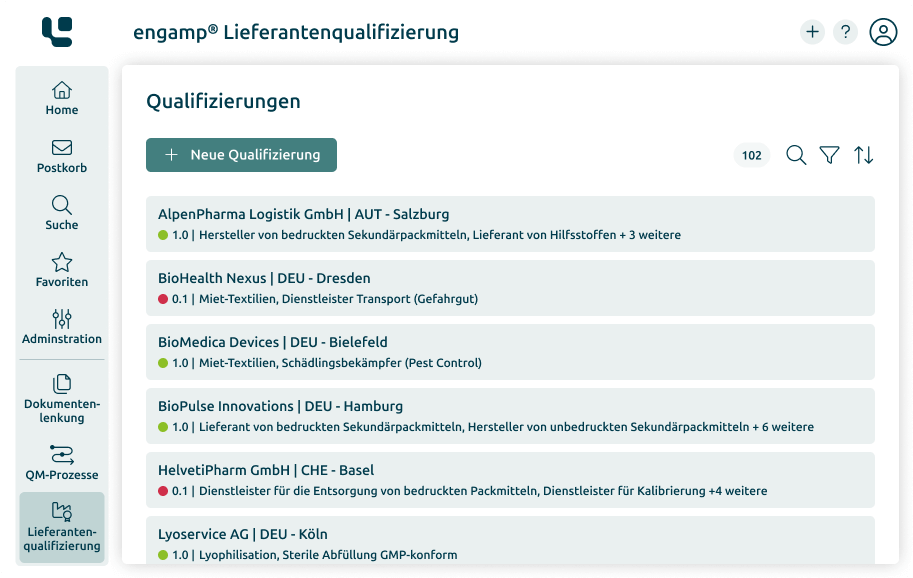
Supplier qualification
Improve the
quality of your suppliers
Ensure the quality of your products and minimise risks in global supply chains through comprehensive supplier qualification. Start your success story today!
-
Always up-to-date supplier status
-
Compliance with specific regulations
-
Increasing efficiency and transparency


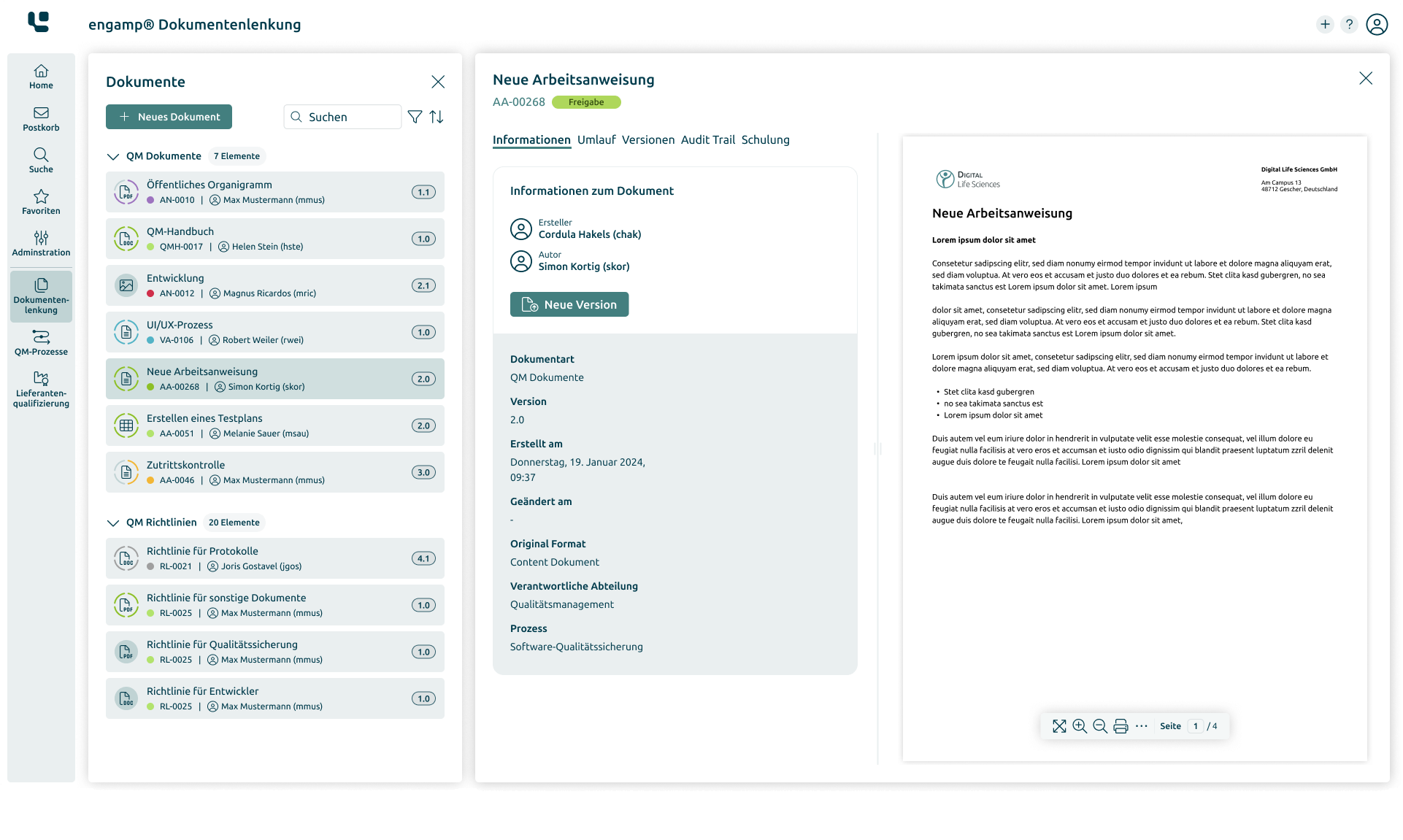
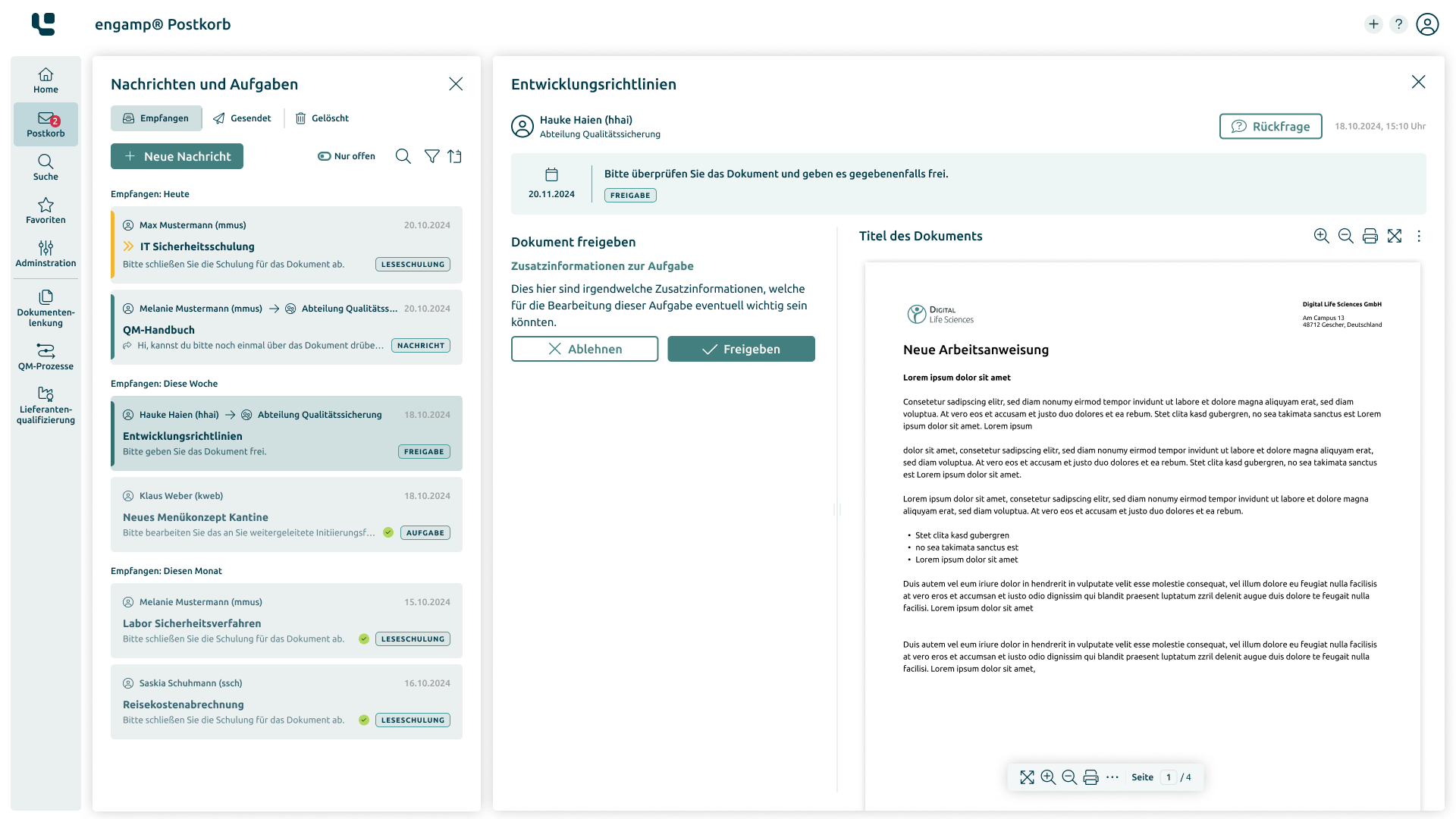
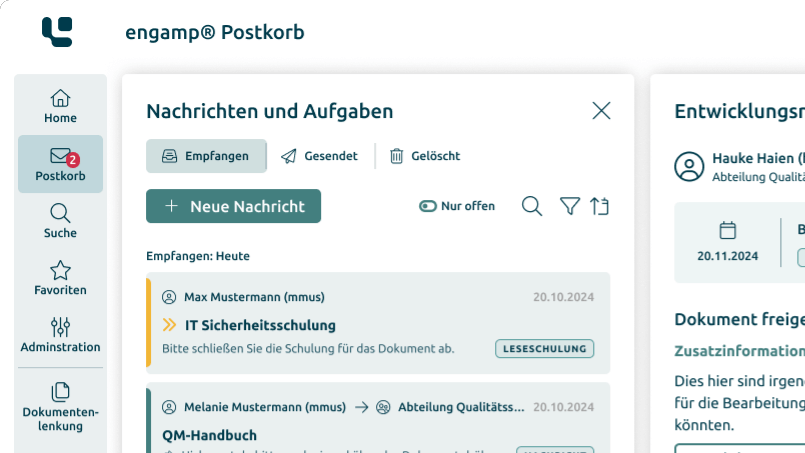
Document Control
Control your documents digitally
Whether SOPs, process descriptions, test specifications or other document types – with the document control software you can create, edit, and sign all these documents digitally. Start your digital transformation today!
-
Manage documents efficiently and securely
-
Adherence to compliance
-
Designing business processes digitally


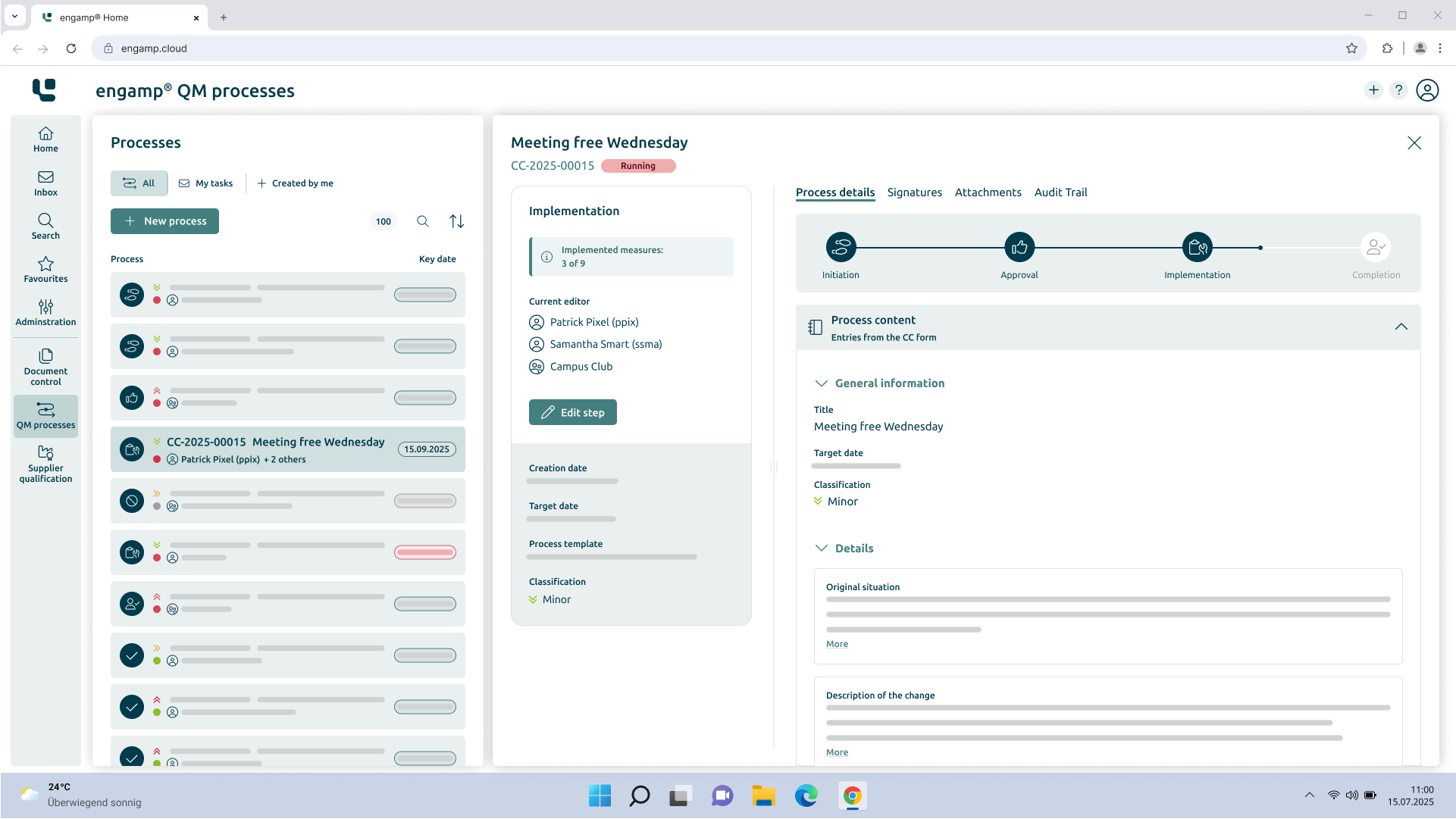
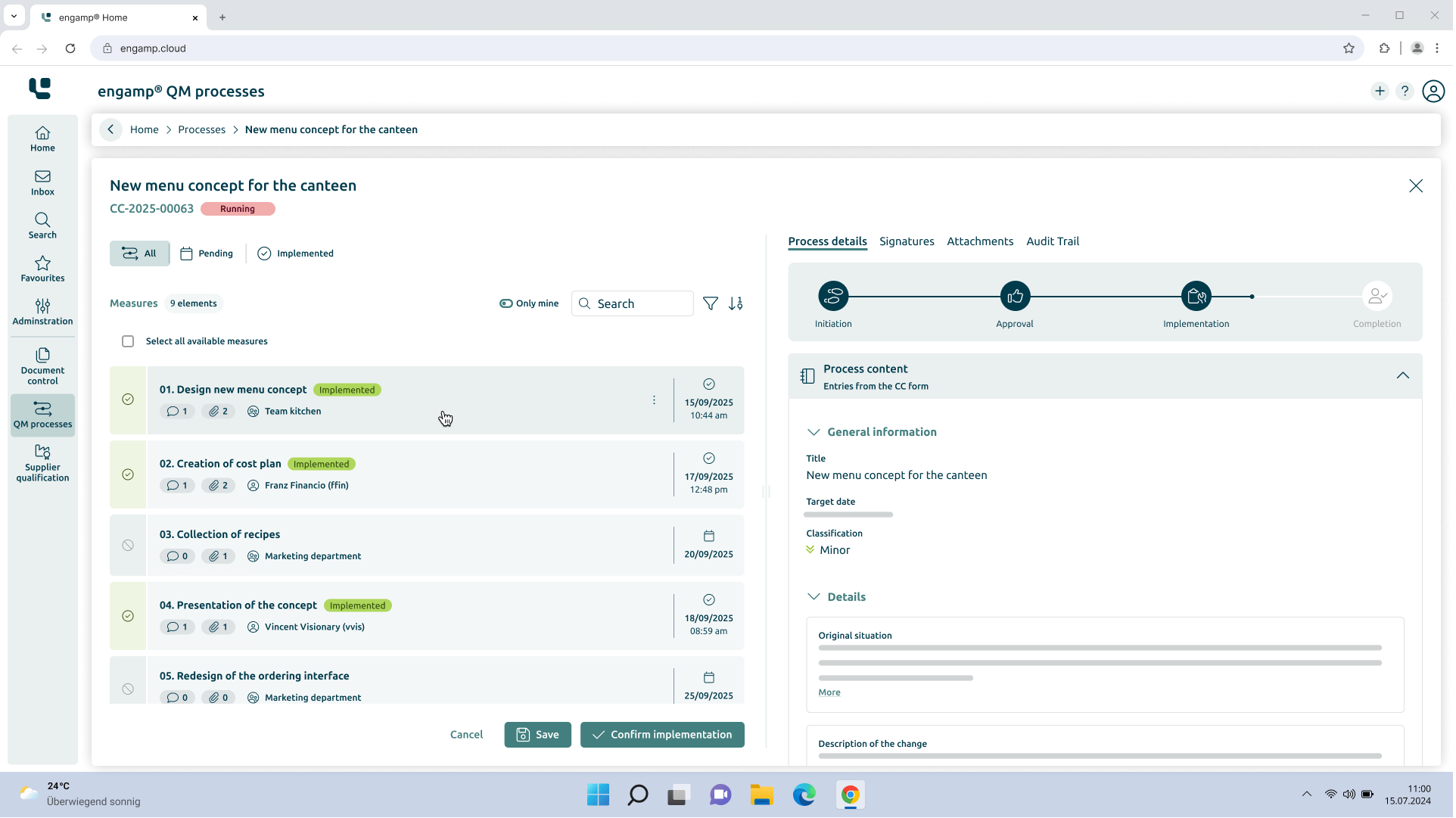
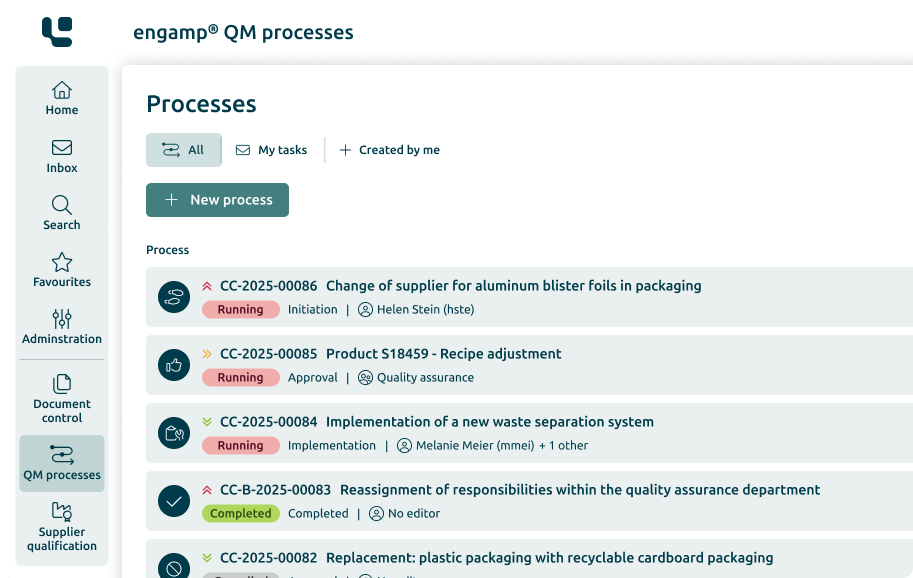
Change Control
Control your
Change processes
The change management software optimises your production-related QM processes through automated workflows – for greater transparency and traceability. Shape your future digitally – start now!
-
Control of the change processes
-
Increasing data security
-
Consideration of regulations

Advantages of engamp QMS
Some advantages of the engamp QM software
You have already recognised that digital quality management speeds up your processes, supports compliance with regulatory requirements and at the same time raises data security to a new level. But why exactly should you choose Digital Life Sciences' solutions and services?
Customers Choice
In addition to classic on-premise operation, we also offer you the alternative of using our suite in the cloud.
Digital Management
Manage documents such as SOPs, test instructions, hygiene plans, and contracts in a structured and audit-proof manner.
Completion at the touch of a button
Release processes, control, versioning – all done with just a few clicks.
Increased data security
Your data is comprehensively protected by differentiated authorisation concepts and technical security measures.
Minimisation of process runtimes
Optimised workflows reduce processing times when creating, reviewing, releasing and distributing your specification documents.
Reduction of resources
Less paper, fewer manual steps – more sustainability and efficiency.
QMS regulations
Regulatory requirements for QM software
The management of controlled documents is subject to a large number of regulatory requirements that ensure that documentation processes are carried out in a traceable, secure manner, and in compliance with international standards. Some of the most important regulations that must be observed in document control are listed below:
ISO 9001:2015, Chapter 7.5
✔ Requirements for the documentation of information as part of a quality management system (QMS).
✔ Ensuring the availability, protection, control, and updating of documented information.
ISO 13485:2016, Chapter 4
✔ Specific requirements for document control for manufacturers of medical products.
✔ Requires audit-proof management of quality documents.
FDA 21 CFR Part 11
✔ Regulations of the US Food and Drug Administration (FDA) for the use of electronic records and signatures.
✔ Requirements for authenticity, integrity, availability, and traceability of electronic documents.
EU-GMP guideline, Annex 11
✔ Requirements for computerised systems to ensure data integrity in the pharmaceutical industry.
✔ Refers to software validation, audit trails, and electronic signatures.
WHO Guidelines on Good Practices in Data and Record Management
✔ World Health Organization guidelines for ensuring data integrity, traceability, and authenticity in regulated industries.
EFG Votum V11002: Requirements regarding the electronic data storage
✔ Defines standards for the long-term archiving of electronic data, taking into account security, integrity, and access protection.
EFG Votum V11003: Requirements regarding electronic signatures and initials
✔ Specifies the criteria that electronic signatures must meet in order to be legally binding and tamper-proof.
FAQ QMS
Frequently asked questions (FAQ's)
about the Quality Management System (QMS)
Expand FAQ's
A QMS is not only essential in the life sciences sector (e.g. pharmaceuticals, medical technology, food supplements, cosmetics), but also in many other sectors with complex processes. These include:
- Service sector (increasing significance due to process standardisation)
- Laboratories: ISO 17025
- Automotive industry: IATF 16949
- Medical product manufacturer: ISO 13485
These specialised standards are based on ISO 9001, but are expanded to include industry-specific requirements. In all cases, a QMS serves to optimise quality, efficiency, and risk minimisation.
An effective QMS supports companies by:
- Improved market access: Fulfillment of industry-specific admission criteria
- Reduced error costs: Early detection of defects reduces quality costs
- Sustainable optimisation: Standardisation of proven processes
- Knowledge management: Easier onboarding training of new employees
- Process transparency: Documentation and control of operational processes
- Involvement of employees: Quality awareness at all levels
A QM system is a formalised system for ensuring and continuously improving the quality of products and services. It includes:
- Processes and process instructions
- SOPs and CAPA processes
- Audit management
- Measures for compliance with legal regulations
The aim is to increase process efficiency, reduce risks, and increase customer satisfaction. The integration of an eQMS (electronic QM system) also supports the digital transformation and enables in-depth data analysis for continuous improvement.
The GxP guidelines set comprehensive requirements for quality management in regulated industries. According to §3 of the German Ordinance on the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients (AMWHV), a company must implement a quality management system (QMS) that corresponds to the type and scope of its activities. Key aspects include:
- Involvement of the management and all relevant areas
- Competence and equipment of the staff
- Compliance with good manufacturing practice (GMP) or good professional practice (GFP)
- Documentation and proof of proper functioning of the system
These requirements apply in particular to companies in the GxP environment in order to ensure consistent product quality and compliance with regulatory requirements.
The engamp QM software offers a modular, validated system with the following advantages:
- Digital management of SOPs, test specifications, contracts, and much more.
- Automated document control with signature workflow
- High data and access security
- Reduction of paper consumption and process runtimes
- Seamless integration into LIMS and ERP systems
The solution is also configurable and is continuously developed as standard software – with a strong validation basis in accordance with GAMP 5.
Consistent quality management...
- ...ensures compliance with standards such as FDA 21 CFR Part 11 or EU GMP.
- ...reduces deviations and errors through structured processes.
- ...promotes transparency, traceability and audit compliance.
- ... supports targeted audit preparations through systematic documentation.
- ...strengthens confidence in products and services, which increases competitiveness.
Yes. The software solutions from Digital Life Sciences have been successfully validated in numerous pharmaceutical companies. They were, among other things:
- TÜV-certified according to ISO 9001 and ISO 13485
- inspected by regional councils
- audited in accordance with GAMP 5, both by mail and on site
This means that customers are ideally prepared for supplier audits, inspections by authorities, and certification audits.
QM software is a digital system for planning, controlling, and monitoring quality-related processes. It includes, among other things:
- Document management
- Deviation control
- Change management
- CAPA management
With suitable QM software, regulatory requirements such as ISO 13485, ISO 9001, ISO 17025, FDA 21 CFR Part 11 and MDR 2017/745 can be reliably implemented. This also includes the audit-proof management of:
- SOPs (standard operating procedures)
- Quality management manuals
- Test reports and CAPA evidence
Digitalisation takes quality management to a new level:
- Centralised document management: Transparent versioning and access control
- Standardised processes: Fewer errors, better traceability
- Integration: Compatibility with existing IT systems
- Efficient training processes: e.g. through standardised QM presentations
- Legally compliant technical documentation: Particularly relevant for regulated industries
A digitalised QMS is the key to sustainable quality assurance in an increasingly complex and regulated world.
Your advantages
All features in a single system
Our software solutions specifically support companies in the life sciences sector, promoting growth and increasing efficiency.
-
Receive outstanding service
-
Make well-founded, strategic decisions
-
Optimise your processes and workflows
Monthly Downloads
Page Speed
Expert-built templates
Load Time
Contact QMS software
Are you ready to optimise your business processes?
Turn potential into success – use our pioneering QM cloud solutions!
Would you like us to call you back?
We will be happy to call you back. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.
Are you interested in a presentation?
We are happy to offer you a presentation free of charge. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.