Your
holistic
all-in-one
integrated
Software for success in quality management
Your holistic all-in-one integrated Software for success in quality management
Experience the future of document and quality management with our new engamp® product line – our specifically developed, GxP-compliant software solutions. engamp® ensures secure and legally compliant archiving and enables consistent, efficient control of your quality management processes.
- Efficient
- Compliant with standards
- Modularly expandable
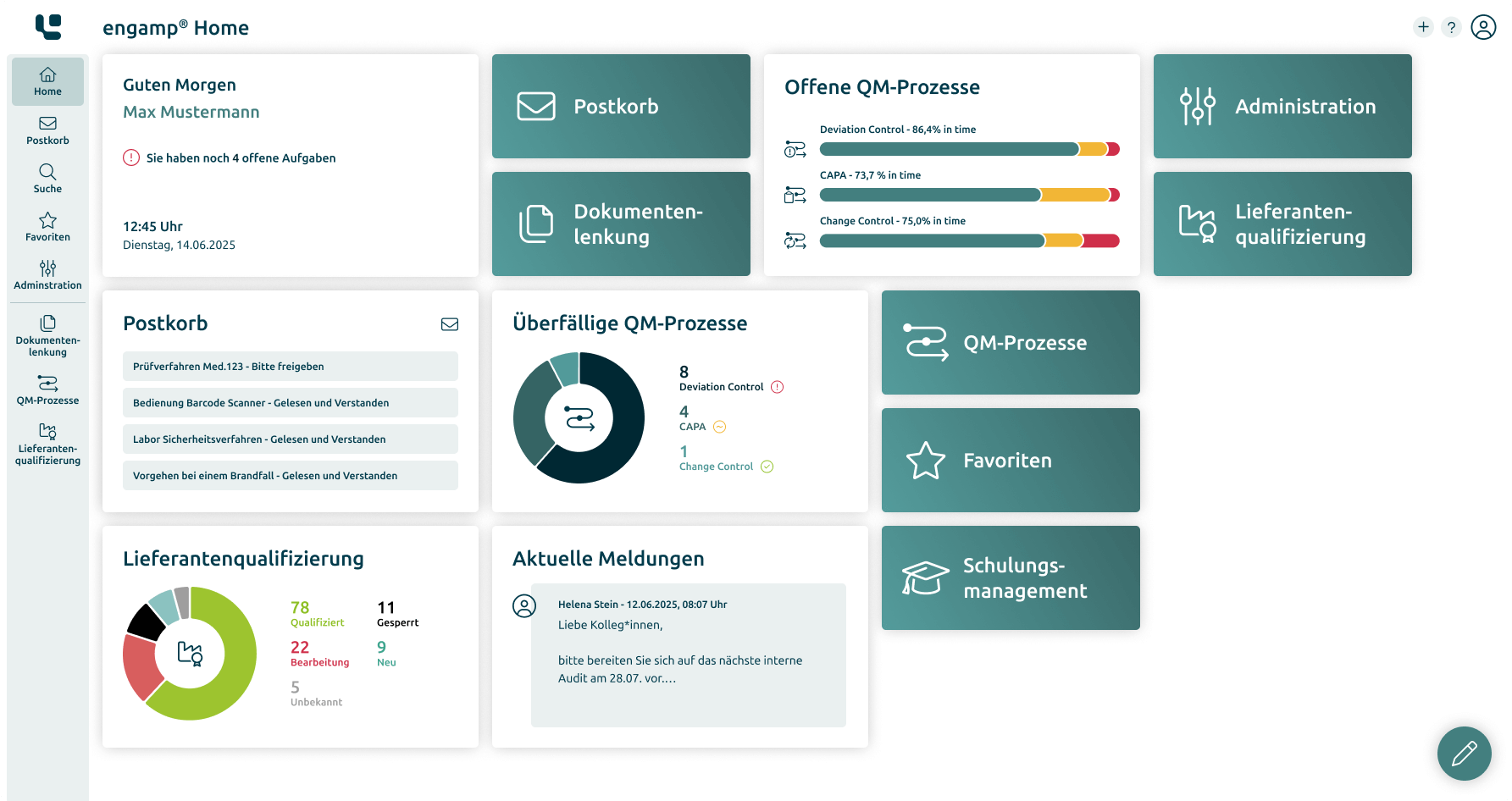
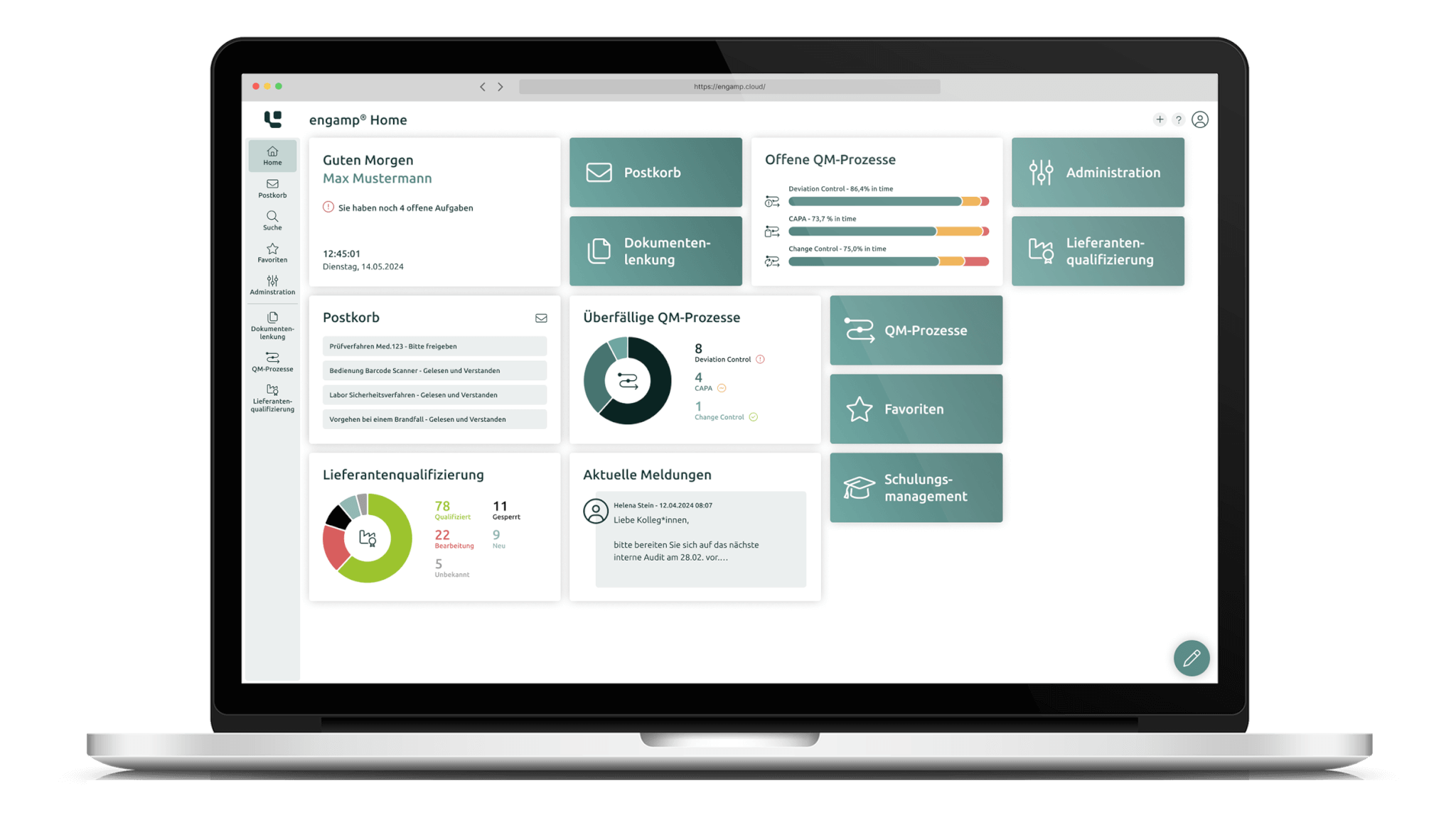
Digital solutions for controlled processes – secure and scalable
Our GxP-compliant software solutions are specifically designed to archive your documents securely and in compliance with the law and to enable efficient control of quality management processes. We place the highest value on quality and reliability.
Document control and archiving
Centralise and secure your documents in accordance with GxP regulations. Use electronic signatures and audit trails for maximum data security and legal compliance.
Comprehensive reporting and analysis
Generate meaningful reports and statistics in the shortest possible time. Automated analyses allow you to make informed decisions faster, minimise manual effort, and reduce your process costs.
Increased efficiency thanks to digital QM processes
Optimise important quality management processes such as change processes digitally. Increase efficiency and transparency in your company.
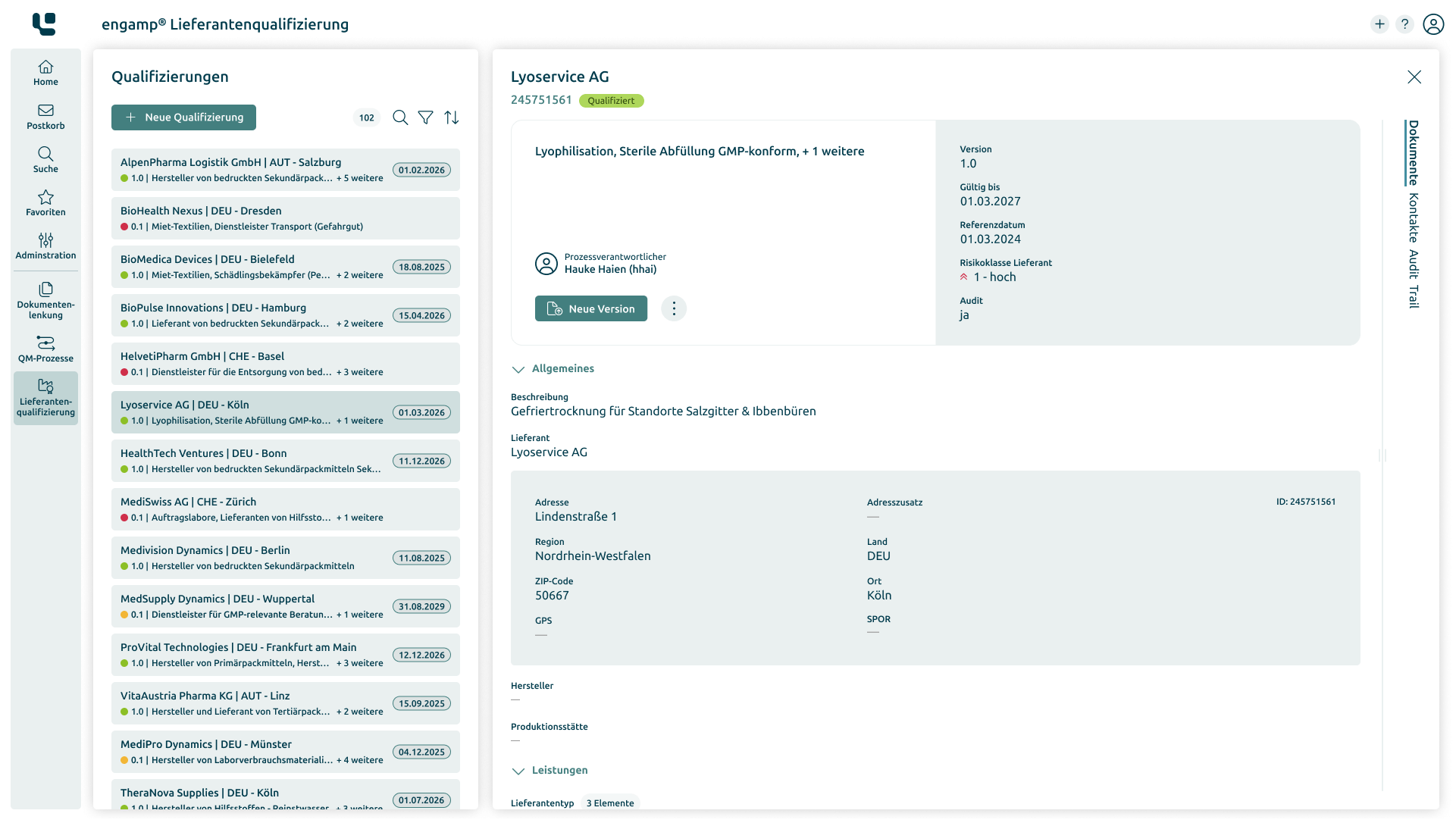
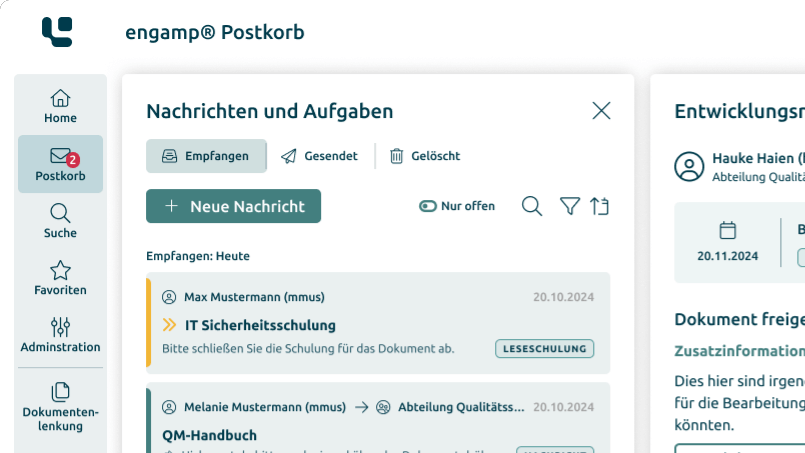
Supplier Qualification
Improve the
quality of your suppliers
Ensure the quality of your products and minimise risks in global supply chains through comprehensive supplier qualification. Start your success story today!
-
Always up-to-date supplier status
-
Compliance with specific regulations
-
Increasing efficiency and transparency
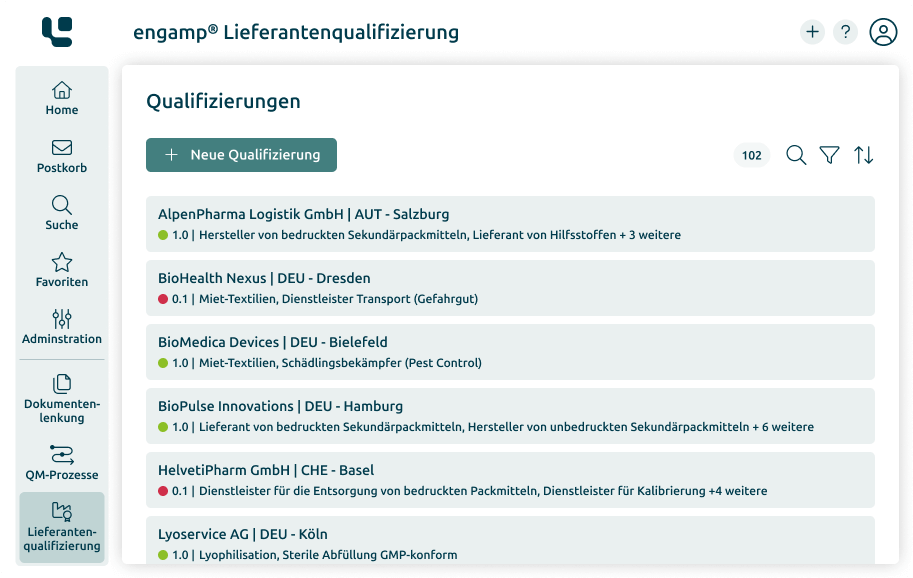
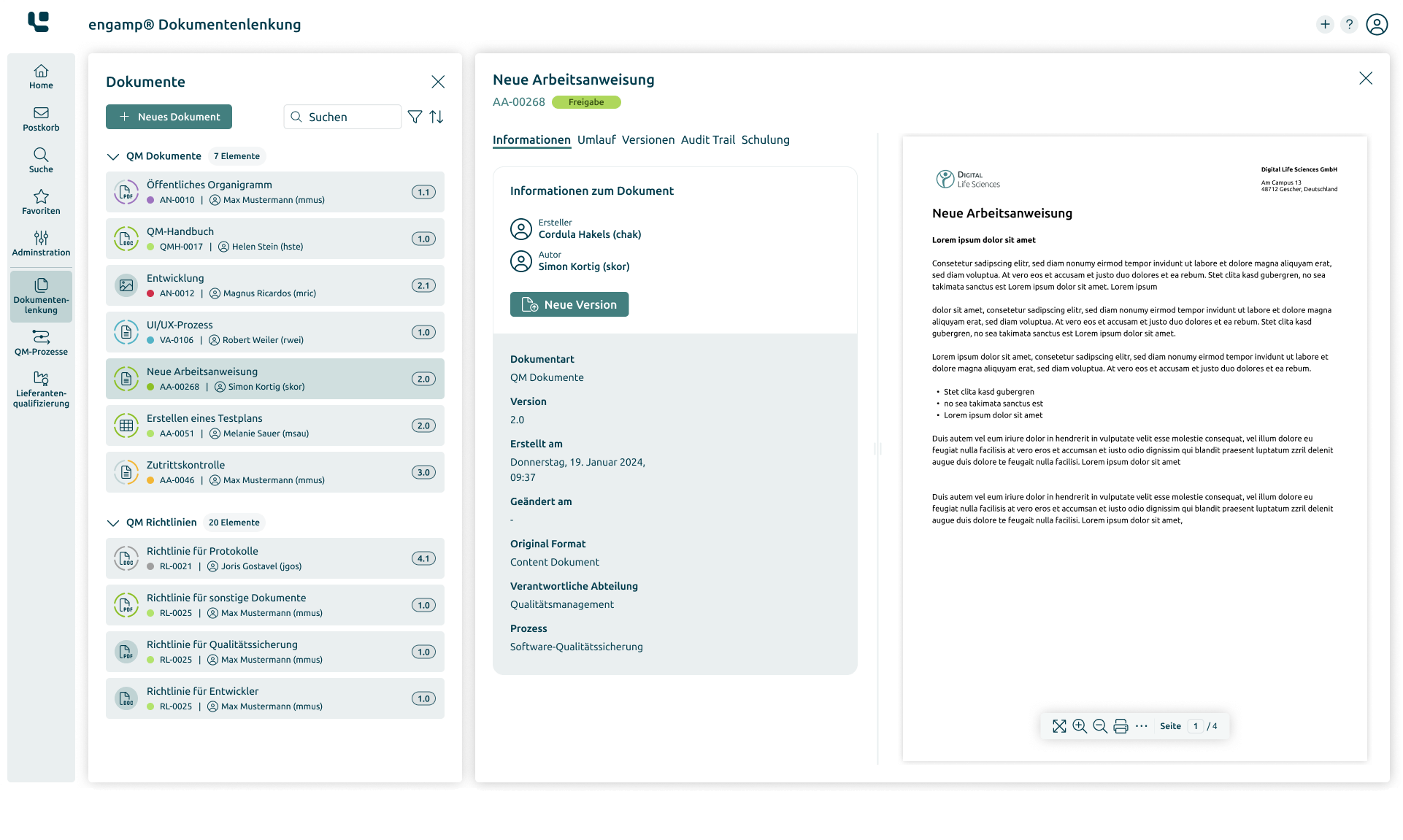
Document Control
Manage your documents digitally
Whether SOPs, process descriptions, test specifications or other document types – with the Document Control software you can create, edit, and sign all these documents digitally. Start your digital transformation today!
-
Manage documents efficiently and securely
-
Adherence to compliance
-
Designing business processes digitally
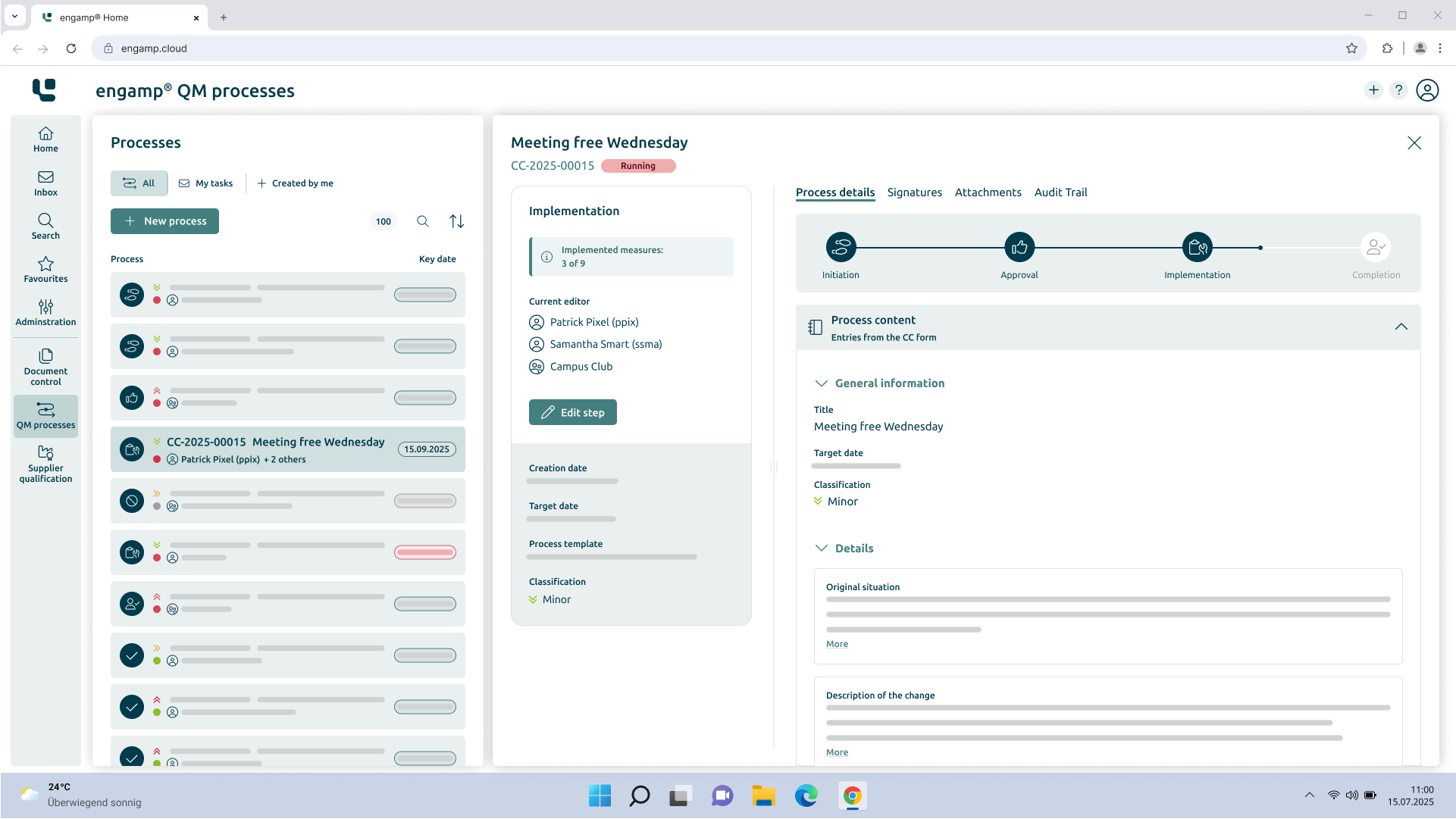
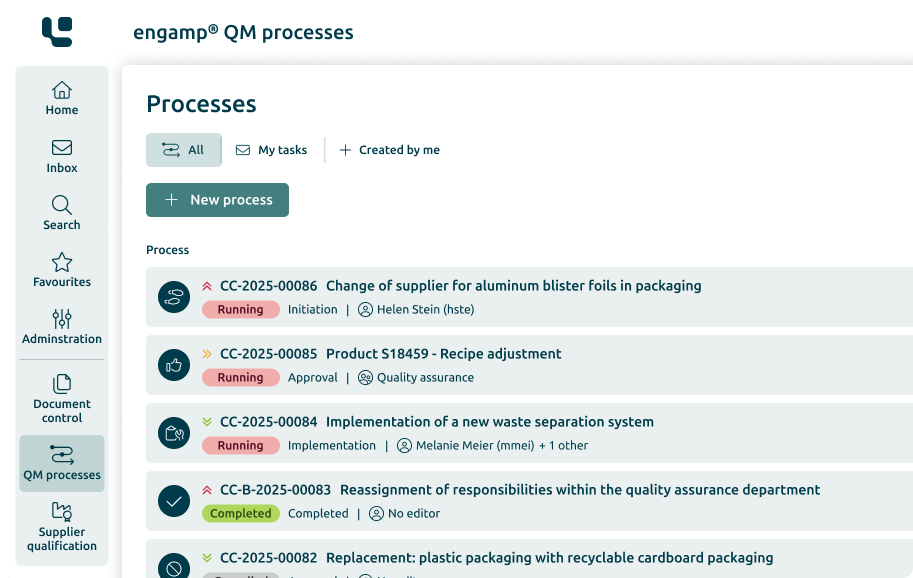
Change Control
Control your
Change processes
The change management software optimises your production-related QM processes through automated workflows – for greater transparency and traceability. Shape your future digitally - start now!
-
Control of the change processes
-
Increasing data security
-
Consideration of regulations
Integrations with 3rd party plugins
Integrate effortlessly with your favorite apps. Enhance functionality, boost efficiency, and streamline workflows.
Stay informed!
Would you like to be regularly informed about current topics of Digital Life Sciences? Register now and benefit – without annoying spam!
The brand engamp®
Our engamp® eQMS suite supports you with integrated solutions for digital document management, training management, and continuous process improvement. It fulfills all relevant regulatory requirements, significantly reduces the audit effort, and sustainably increases the efficiency of your quality management.
Customers Choice
For an optimal operating mode of our software solutions, we offer you the use of engamp® in the cloud as an alternative to classic on-premise operation.
Automation
engamp® stands for the automation of processes and workflows made possible by cloud solutions, thereby increasing efficiency and productivity.
Security
engamp® stands for high security and the protection of sensitive data, which is of the utmost importance for companies in the life science sector.
Performance
engamp® stands for the outstanding performance and speed of cloud services that provide a stable and secure platform for digital quality management solutions.
Mobility
engamp® stands for mobility and flexibility as cloud-based solutions are accessible anytime and anywhere, which supports high adaptability and modern ways of working.
Commitment
engamp® stands for the deep commitment and dedication of Digital Life Sciences GmbH to provide excellent solutions that are specifically tailored to the needs of the life sciences industry.
Current News
Discover the latest trends, tips, and insights in our world. Get the knowledge you need to grow your business and increase productivity.
What do our customers say about us?
Find out how companies have redesigned their processes with our software – with measurable successes in efficiency, quality, and growth.
Digital Life Sciences GmbH supported us with professionalism and expertise in record time when we switched to a new electronic quality management system.
SCHOTT AG
Christine Strubel
Head of Management Systems
Quality & EHS


We have been a customer from the very beginning and Digital Life Sciences supports us reliably and sustainably with its products, helping us to further develop our QMS.
MEDICE Arzneimittel Pütter
Silke Schwiertz
Head of QM/QA/QP


Together with the team of Digital Life Sciences, we implemented our new GMP-compliant DMS and training tool in no time. [...] The team says: Thank you and we'd be pleased to do it again!"
HENNIG ARZNEIMITTEL
Meike Murawski
QA Manager Validation and Projects


Digital Life Sciences has helped us to make our paper-based processes more digital. […] Today, we work with real digital documents that we can distribute with just a few clicks.
Corden Pharma Switzerland LLC
Dr.-Ing. Witali Blum
Qualification Expert


Thanks to the project team of Digital Life Sciences, the new DMS was implemented quickly in just three months.
METASYS Medizintechnik GmbH
Dr. Florian Meischl
Head of Innovation and
Project Management


All customer testimonials
DEMO QM SOFTWARE
Are you ready to optimise your business processes?
Turn potential into success – Use our pioneering QM cloud solutions!
Would you like us to call you back?
We will be happy to call you back. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.
Are you interested in a presentation?
We are happy to offer you a presentation free of charge. Please fill out the form below. One of our experts will then get in touch with you as soon as possible.